24S-hydroxycholesterol effects on lipid metabolism genes are modeled in traumatic brain injury
- PMID: 20053345
- PMCID: PMC2826556
- DOI: 10.1016/j.brainres.2009.12.080
24S-hydroxycholesterol effects on lipid metabolism genes are modeled in traumatic brain injury
Abstract
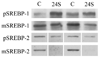
Membrane damage during traumatic brain injury (TBI) alters the brain homeostasis of cholesterol and other lipids. Cholesterol 24S-hydroxylase (Cyp46) is a cholesterol metabolic enzyme that is increased after TBI. Here, we systematically examined the effects of the enzymatic product of Cyp46, 24S-hydroxycholesterol, on the cholesterol regulatory genes, SREBP-1 and 2, their posttranslational regulation, and their effects on gene transcription. 24S-hydroxycholesterol increased levels of SREBP-1 mRNA and full-length protein but did not change levels of cleaved SREBP-1, consistent with the role of 24-hydroxycholesterol as an LXR agonist. In contrast, 24S-hydroxycholesterol decreased levels of LXR-independent SREBP-2 mRNA, full-length protein, and SREBP-2 active cleavage product. We examined the downstream effects of changes to these lipid regulatory factors by studying cholesterol and fatty acid synthesis genes. In neuroblastoma cells, 24S-hydroxycholesterol decreased mRNA levels of the cholesterol synthesis genes HMG CoA reductase, squalene synthase, and FPP synthase but did not alter levels of the mRNA of fatty acid synthesis genes acetyl CoA carboxylase or fatty acid synthase. After TBI, as after 24S-hydroxycholesterol treatment in vitro, SREBP-1 mRNA levels were increased while SREBP-2 mRNA levels were decreased. Also similar to the in vitro results with 24S-hydroxycholesterol, HMG CoA reductase and squalene synthase mRNA levels were significantly decreased. Fatty acid synthase mRNA levels were not altered but acetyl CoA carboxylase mRNA levels were significantly decreased. Thus, changes to transcription of cholesterol synthesis genes after TBI were consistent with increases in Cyp46 activity, but changes to fatty acid synthesis genes must be regulated by other mechanisms.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Cortical injury increases cholesterol 24S hydroxylase (Cyp46) levels in the rat brain.J Neurotrauma. 2008 Sep;25(9):1087-98. doi: 10.1089/neu.2007.0444. J Neurotrauma. 2008. PMID: 18729719 Free PMC article.
-
25-Hydroxycholesterol-3-sulfate regulates macrophage lipid metabolism via the LXR/SREBP-1 signaling pathway.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1369-79. doi: 10.1152/ajpendo.90555.2008. Epub 2008 Oct 14. Am J Physiol Endocrinol Metab. 2008. PMID: 18854425 Free PMC article.
-
The effect of 24S-hydroxycholesterol on cholesterol homeostasis in neurons: quantitative changes to the cortical neuron proteome.J Proteome Res. 2008 Apr;7(4):1606-14. doi: 10.1021/pr7006076. Epub 2008 Feb 28. J Proteome Res. 2008. PMID: 18303831 Free PMC article.
-
24S-hydroxycholesterol and cholesterol-24S-hydroxylase (CYP46A1) in the retina: from cholesterol homeostasis to pathophysiology of glaucoma.Chem Phys Lipids. 2011 Sep;164(6):496-9. doi: 10.1016/j.chemphyslip.2011.04.006. Epub 2011 Apr 21. Chem Phys Lipids. 2011. PMID: 21531213 Review.
-
The impairment of cholesterol metabolism in Huntington disease.Biochim Biophys Acta. 2015 Aug;1851(8):1095-105. doi: 10.1016/j.bbalip.2014.12.018. Epub 2015 Jan 14. Biochim Biophys Acta. 2015. PMID: 25596342 Review.
Cited by
-
Intracellular cholesterol homeostasis and amyloid precursor protein processing.Biochim Biophys Acta. 2010 Aug;1801(8):853-9. doi: 10.1016/j.bbalip.2010.03.004. Epub 2010 Mar 19. Biochim Biophys Acta. 2010. PMID: 20304094 Free PMC article. Review.
-
Cholesterol metabolism and brain injury in neonatal encephalopathy.Pediatr Res. 2021 Jul;90(1):37-44. doi: 10.1038/s41390-020-01218-3. Epub 2020 Oct 26. Pediatr Res. 2021. PMID: 33106607 Free PMC article. Review.
-
Food Restriction Counteracts Dexamethasone-Induced Downregulation of Genes Involved in Cholesterol Homeostasis in Rat Brain during Aging.Brain Sci. 2022 Sep 26;12(10):1297. doi: 10.3390/brainsci12101297. Brain Sci. 2022. PMID: 36291231 Free PMC article.
-
The Pathological Effects of Circulating Hydrophobic Bile Acids in Alzheimer's Disease.J Alzheimers Dis Rep. 2023 Mar 6;7(1):173-211. doi: 10.3233/ADR-220071. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 36994114 Free PMC article. Review.
-
Treatment with efavirenz extends survival in a Creutzfeldt-Jakob disease model by regulating brain cholesterol metabolism.JCI Insight. 2025 Jun 19;10(14):e190296. doi: 10.1172/jci.insight.190296. eCollection 2025 Jul 22. JCI Insight. 2025. PMID: 40540390 Free PMC article.
References
-
- Abildayeva K, Jansen PJ, Hirsch-Reinshagen V, Bloks VW, Bakker AH, Ramaekers FC, de Vente J, Groen AK, Wellington CL, Kuipers F, Mulder M. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J Biol Chem. 2006;281:12799–12808. - PubMed
-
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem. 2004;279:52772–52780. - PubMed
-
- Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. - PubMed
-
- Bogdanovic N, Bretillon L, Lund EG, Diczfalusy U, Lannfelt L, Winblad B, Russell DW, Bjorkhem I. On the turnover of brain cholesterol in patients with Alzheimer's disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neuroscience Letters. 2001;314:45–48. - PubMed
-
- Brown J, III, Theisler C, Silberman S, Magnuson D, Gootardi-Littell N, Lee JM, Yager D, Crowley J, Sambamuri K, Rahman MM, Reiss AB, Eckman CB, Wolozin B. Differential expression of cholesterol hydroxylases in Alzheimer's disease. Journal of Biological Chemistry. 2004;279:34674–34681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

