Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium
- PMID: 20053363
- PMCID: PMC2879464
- DOI: 10.1016/j.mvr.2009.12.010
Quantitative distribution and colocalization of non-muscle myosin light chain kinase isoforms and cortactin in human lung endothelium
Abstract
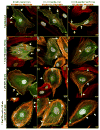
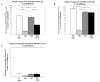
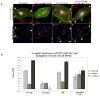
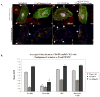
Vascular barrier regulation is intimately linked to alterations in the distribution and configuration of the endothelial cell (EC) cytoskeleton in response to angiogenic and edemagenic agonists. Critical actin cytoskeletal rearrangement includes spatially directed increases in myosin light chain (MLC) phosphorylation, catalyzed by Ca(2+)/calmodulin-dependent non-muscle myosin light chain kinase variants (nmMLCK1- and -2), as well as association of nmMLCK with the actin-binding protein, cortactin. As these associations have proven difficult to quantify in a spatially specific manner, we now describe the utility of intensity correlation image analysis and the intensity correlation quotient (ICQ) to quantify colocalization in fixed and live cell imaging assays in human pulmonary artery EC. From baseline ICQ values averaging 0.216 reflecting colocalization of cortactin-DsRed with EGFP-nmMLCK fusion proteins in resting EC, thrombin-induced EC contraction significantly reduced cortactin-DsRed-EGFP-nmMLCK colocalization (nmMLCK1: ICQ=0.118; nmMLCK2: ICQ=0.091) whereas the potent EC barrier-protective agonist, sphingosine 1-phosphate (S1P), significantly increased nmMLCK-cortactin colocalization within lamellipodia (nmMLCK1: ICQ=0.275; nmMLCK2: ICQ=0.334). Over-expression of a cortactin-DsRed mutant fusion protein lacking the SH3 domain, known to be essential for cortactin-nmMLCK association, reduced baseline and S1P-mediated live cell colocalization with each nmMLCK variant (nmMLCK1: ICQ=0.160; nmMLCK2: ICQ=0.157). Similarly, expression of a truncated EGFP-nmMLCK2 mutant lacking cortactin- and actin-binding domains, markedly reduced basal localization in lamellipodia and abolished colocalization with cortactin-DsRed in lamellipodia after S1P (ICQ=-0.148). These data provide insights into the molecular basis for vascular barrier-regulatory cytoskeletal responses and support the utility of sophisticated imaging analyses and methodological assessment to quantify the critical nmMLCK and cortactin interaction during vascular barrier regulation.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Proline-rich region of non-muscle myosin light chain kinase modulates kinase activity and endothelial cytoskeletal dynamics.Microvasc Res. 2014 Sep;95:94-102. doi: 10.1016/j.mvr.2014.07.007. Epub 2014 Jul 27. Microvasc Res. 2014. PMID: 25072537 Free PMC article.
-
Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase.J Biol Chem. 2004 Jun 4;279(23):24692-700. doi: 10.1074/jbc.M313969200. Epub 2004 Mar 31. J Biol Chem. 2004. PMID: 15056655
-
Abl tyrosine kinase phosphorylates nonmuscle Myosin light chain kinase to regulate endothelial barrier function.Mol Biol Cell. 2010 Nov 15;21(22):4042-56. doi: 10.1091/mbc.E09-10-0876. Epub 2010 Sep 22. Mol Biol Cell. 2010. PMID: 20861316 Free PMC article.
-
Novel role for non-muscle myosin light chain kinase (MLCK) in hyperoxia-induced recruitment of cytoskeletal proteins, NADPH oxidase activation, and reactive oxygen species generation in lung endothelium.J Biol Chem. 2012 Mar 16;287(12):9360-75. doi: 10.1074/jbc.M111.294546. Epub 2012 Jan 4. J Biol Chem. 2012. PMID: 22219181 Free PMC article.
-
Cortactin branches out: roles in regulating protrusive actin dynamics.Cell Motil Cytoskeleton. 2008 Sep;65(9):687-707. doi: 10.1002/cm.20296. Cell Motil Cytoskeleton. 2008. PMID: 18615630 Free PMC article. Review.
Cited by
-
P2Y receptors as regulators of lung endothelial barrier integrity.J Cardiovasc Dis Res. 2011 Jan;2(1):14-22. doi: 10.4103/0975-3583.78582. J Cardiovasc Dis Res. 2011. PMID: 21716747 Free PMC article.
-
Cortactin in Lung Cell Function and Disease.Int J Mol Sci. 2022 Apr 21;23(9):4606. doi: 10.3390/ijms23094606. Int J Mol Sci. 2022. PMID: 35562995 Free PMC article. Review.
-
The Significant Role of c-Abl Kinase in Barrier Altering Agonists-mediated Cytoskeletal Biomechanics.Sci Rep. 2018 Jan 17;8(1):1002. doi: 10.1038/s41598-018-19423-w. Sci Rep. 2018. PMID: 29343719 Free PMC article.
-
Imatinib Alters Agonists-mediated Cytoskeletal Biomechanics in Lung Endothelium.Sci Rep. 2017 Oct 26;7(1):14152. doi: 10.1038/s41598-017-14722-0. Sci Rep. 2017. PMID: 29075042 Free PMC article.
-
Septin-2 mediates airway epithelial barrier function in physiologic and pathologic conditions.Am J Respir Cell Mol Biol. 2011 Jul;45(1):120-6. doi: 10.1165/rcmb.2010-0235OC. Epub 2010 Sep 24. Am J Respir Cell Mol Biol. 2011. PMID: 20870893 Free PMC article.
References
-
- Birukov KG, et al. Differential regulation of alternatively spliced endothelial cell myosin light chain kinase isoforms by p60(Src) J Biol Chem. 2001;276:8567–73. - PubMed
-
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. Journal of Microscopy. 2006;224:213–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

