Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick
- PMID: 20053388
- PMCID: PMC2842458
- DOI: 10.1016/j.bone.2009.12.018
Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick
Abstract
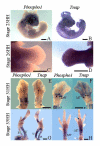
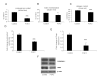
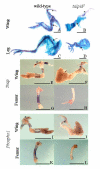
PHOSPHO1 is a bone-specific phosphatase implicated in the initiation of inorganic phosphate generation for matrix mineralization. The control of mineralization is attributed to the actions of tissue-nonspecific alkaline phosphatase (TNAP). However, matrix vesicles (MVs) containing apatite crystals are present in patients with hypophosphatasia as well as TNAP null (Akp2(-/-)) mice. It is therefore likely that other phosphatases work with TNAP to regulate matrix mineralization. Although PHOSPHO1 and TNAP expression is associated with MVs, it is not known if PHOSPHO1 and TNAP are coexpressed during the early stages of limb development. Furthermore, the functional in vivo role of PHOSPHO1 in matrix mineralization has yet to be established. Here, we studied the temporal expression and functional role of PHOSPHO1 within chick limb bud mesenchymal micromass cultures and also in wild-type and talpid(3) chick mutants. These mutants are characterized by defective hedgehog signalling and the absence of endochondral mineralization. The ability of in vitro micromass cultures to differentiate and mineralize their matrix was temporally associated with increased expression of PHOSPHO1 and TNAP. Comparable changes in expression were noted in developing embryonic legs (developmental stages 23-36HH). Micromass cultures treated with lansoprazole, a small-molecule inhibitor of PHOSPHO1 activity, or FGF2, an inhibitor of chondrocyte differentiation, resulted in reduced alizarin red staining (P<0.05). FGF2 treatment also caused a reduction in PHOSPHO1 (P<0.001) and TNAP (P<0.001) expression. Expression analysis by whole-mount RNA in situ hybridization correlated with qPCR micromass data and demonstrated the existence of a tightly regulated pattern of Phospho1 and Tnap expression which precedes mineralization. Treatment of developing embryos for 5 days with lansoprazole completely inhibited mineralization of all leg and wing long bones as assessed by alcian blue/alizarin red staining. Furthermore, long bones of the talpid(3) chick mutant did not express Phospho1 or Tnap whereas flat bones mineralized normally and expressed both phosphatases. In conclusion, this study has disclosed that PHOSPHO1 expression mirrors that of TNAP during embryonic bone development and that PHOSPHO1 contributes to bone mineralization in developing chick long bones.
Keywords: Alkaline Phosphatase; PHOSPHO1; chondrocyte differentiation; mineralization; talpid3.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures



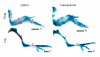
Similar articles
-
The role of phosphatases in the initiation of skeletal mineralization.Calcif Tissue Int. 2013 Oct;93(4):299-306. doi: 10.1007/s00223-012-9672-8. Epub 2012 Nov 27. Calcif Tissue Int. 2013. PMID: 23183786 Free PMC article. Review.
-
Functional involvement of PHOSPHO1 in matrix vesicle-mediated skeletal mineralization.J Bone Miner Res. 2007 Apr;22(4):617-27. doi: 10.1359/jbmr.070108. J Bone Miner Res. 2007. PMID: 17227223
-
Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function: a unified model of the mechanisms of initiation of skeletal calcification.J Bone Miner Res. 2011 Feb;26(2):286-97. doi: 10.1002/jbmr.195. Epub 2010 Aug 3. J Bone Miner Res. 2011. PMID: 20684022 Free PMC article.
-
The presence of PHOSPHO1 in matrix vesicles and its developmental expression prior to skeletal mineralization.Bone. 2006 Nov;39(5):1000-1007. doi: 10.1016/j.bone.2006.05.014. Epub 2006 Jul 11. Bone. 2006. PMID: 16837257
-
The mechanism of mineralization and the role of alkaline phosphatase in health and disease.J Nippon Med Sch. 2010 Feb;77(1):4-12. doi: 10.1272/jnms.77.4. J Nippon Med Sch. 2010. PMID: 20154452 Review.
Cited by
-
Injectable hydrogel for sustained delivery of progranulin derivative Atsttrin in treating diabetic fracture healing.Biomaterials. 2023 Oct;301:122289. doi: 10.1016/j.biomaterials.2023.122289. Epub 2023 Aug 25. Biomaterials. 2023. PMID: 37639975 Free PMC article.
-
Role of PHOSPHO1 in Periodontal Development and Function.J Dent Res. 2016 Jul;95(7):742-51. doi: 10.1177/0022034516640246. Epub 2016 Mar 25. J Dent Res. 2016. PMID: 27016531 Free PMC article.
-
Phospho1 deficiency transiently modifies bone architecture yet produces consistent modification in osteocyte differentiation and vascular porosity with ageing.Bone. 2015 Dec;81:277-291. doi: 10.1016/j.bone.2015.07.035. Epub 2015 Jul 29. Bone. 2015. PMID: 26232374 Free PMC article.
-
The role of phosphatases in the initiation of skeletal mineralization.Calcif Tissue Int. 2013 Oct;93(4):299-306. doi: 10.1007/s00223-012-9672-8. Epub 2012 Nov 27. Calcif Tissue Int. 2013. PMID: 23183786 Free PMC article. Review.
-
Icariin Alleviates IL-1β-Induced Matrix Degradation By Activating The Nrf2/ARE Pathway In Human Chondrocytes.Drug Des Devel Ther. 2019 Nov 21;13:3949-3961. doi: 10.2147/DDDT.S203094. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31819369 Free PMC article.
References
-
- Anderson HC. Matrix vesicles and calcification. Current Rheumatol Rep. 2003;5:222–26. - PubMed
-
- Cecil RNA, Anderson HC. Freeze-fracture studies of matrix vesicle calcification in epiphyseal growth plate. Metab Bone Dis Relat Res. 1978;1:89–97.
-
- Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. - PubMed
-
- Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F024347/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/R/00001614/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/R/00003741/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 AR053102/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

