Calcium, cellular aging, and selective neuronal vulnerability in Parkinson's disease
- PMID: 20053445
- PMCID: PMC3235732
- DOI: 10.1016/j.ceca.2009.12.003
Calcium, cellular aging, and selective neuronal vulnerability in Parkinson's disease
Abstract
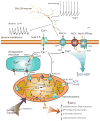
Parkinson's disease (PD) is the second most common neurodegenerative disease in developed countries. The core motor symptoms are attributable to the degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNc). Why these neurons, and other restricted sets of non-dopamine neuron, succumb in PD is not clear. One potential clue has come from the observation that the engagement of L-type Ca2+ channels during autonomous pacemaking elevates the sensitivity of SNc DA neurons to mitochondrial toxins used to create animal models of PD, suggesting that Ca2+ entry is a factor in their selective vulnerability. Epidemiological data also supports a linkage between L-type Ca2+ channels and the risk of developing PD. This review examines the hypothesis that the primary factor driving neurodegenerative changes in PD is the metabolic stress created by sustained Ca2+ entry, particularly in the face of genetic or environmental factors that compromise oxidative defenses or proteostatic competence.
2009 Elsevier Ltd. All rights reserved.
Figures

References
-
- de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–4. - PubMed
-
- de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, Lopez-Pousa S, Manubens-Bertran JM, Alperovitch A, Rocca WA. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:10–5. - PMC - PubMed
-
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–6. - PubMed
-
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–34. - PubMed
-
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

