Flow cytometric analysis of BDE 47 mediated injury to rainbow trout gill epithelial cells
- PMID: 20053465
- PMCID: PMC3321377
- DOI: 10.1016/j.aquatox.2009.11.013
Flow cytometric analysis of BDE 47 mediated injury to rainbow trout gill epithelial cells
Abstract
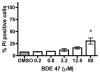
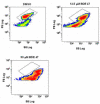
The polybrominated diphenyl ethers (PBDEs) are ubiquitous environmental contaminants whose residues are increasing in fish, wildlife and human tissues. However, relatively little is known regarding the mechanisms of cell injury caused by PBDE congeners in fish. In the present study, we employed flow cytometry-based analyses to understand the onset and mechanisms of cell injury in rainbow trout gill cells (RTgill-W1 cells) exposed to 2,2',4,4'-tetrabromodiphenyl ether (BDE 47). Substantial optimization and validation for flow cytometry protocols were required during assay development for the trout gill cell line. Exposure to micromolar concentrations of BDE 47 elicited a significant loss in RTgill-W1 cell viability that was accompanied by a decrease in NAD(P)H autofluorescence, a marker associated with disruption of cellular redox status. This loss in NAD(P)H content was accompanied by a decrease in nonyl acridine orange fluorescence, indicating mitochondrial membrane lipid peroxidation. Furthermore, low doses of BDE 47 altered cellular forward angle light scatter (FS, a measure of cell diameter or size) and side light scatter properties (SS, a measure of cellular internal complexity), consistent with the early stages of apoptosis. These changes were more pronounced at higher BDE 47 concentrations, which led to an increase in the percentage of cells undergoing frank apoptosis as evidenced by sub-G1 DNA content. Apoptosis was also observed at a relatively low dose (3.2muM) of BDE 47 if cells were exposed for an extended period of time (24h). Collectively, the results of these studies indicate that exposure of rainbow trout gill cells to BDE47 is associated with the induction of apoptosis likely originating from disruption of cellular redox status and mitochondrial oxidative injury. The current report extends observations in other species demonstrating that oxidative stress is an important mechanism of BDE 47 mediated cellular toxicity, and supports the use of oxidative stress-associated biomarkers in assessing the sublethal effects of PBDEs and their replacements in fish. The application of flow cytometry endpoints using fish cell lines should facilitate study of the mechanisms of chemical injury in aquatic species.
Figures






References
-
- Ahmed SA, Gogal RM, Jr., Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–24. - PubMed
-
- Antonsson B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell Biochem. 2004;256-257:141–55. - PubMed
-
- Betts KS. Rapidly rising PBDE levels in North America. Environ. Sci. Technol. 2002;36:50A–52A. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

