Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle
- PMID: 20053628
- PMCID: PMC2900886
- DOI: 10.1093/glycob/cwp203
Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle
Abstract
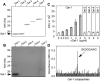
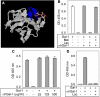
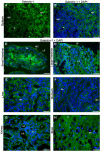
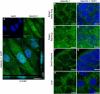
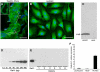
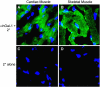
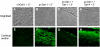
Galectin-1 (Gal-1) is important in immune function and muscle regeneration, but its expression and localization in adult tissues and primary leukocytes remain unclear. To address this, we generated a specific monoclonal antibody against Gal-1, termed alphahGal-1, and defined a sequential peptide epitope that it recognizes, which is preserved in human and porcine Gal-1, but not in murine Gal-1. Using alphahGal-1, we found that Gal-1 is expressed in a wide range of porcine tissues, including striated muscle, liver, lung, brain, kidney, spleen, and intestine. In most types of cells, Gal-1 exhibits diffuse cytosolic expression, but in cells within the splenic red pulp, Gal-1 showed both cytosolic and nuclear localization. Gal-1 was also expressed in arterial walls and exhibited prominent cytosolic and nuclear staining in cultured human endothelial cells. However, human peripheral leukocytes and promyelocytic HL60 cells lack detectable Gal-1 and also showed very low levels of Gal-1 mRNA. In striking contrast, Gal-1 exhibited an organized cytosolic staining pattern within striated muscle tissue of cardiac and skeletal muscle and colocalized with sarcomeric actin on I bands. These results provide insights into previously defined roles for Gal-1 in inflammation, immune regulation and muscle biology.
Figures


References
-
- Ahmed H, Fink NE, Pohl J., Vasta GR. Galectin-1 from bovine spleen: biochemical characterization, carbohydrate specificity and tissue-specific isoform profiles. J Biochem. (Tokyo) 1996;120:1007–1019. - PubMed
-
- Antia R, Ganusov VV, Ahmed R. The role of models in understanding CD8 T-cell memory. Nat Rev Immunol. 2005;5:101–111. - PubMed
-
- Baum LG, Seilhamer JJ, Pang M, Levine WB, Beynon D, Berliner JA. Synthesis of an endogeneous lectin, galectin-1, by human endothelial cells is up-regulated by endothelial cell activation. Glycoconj J. 1995;12:63–68. - PubMed
-
- Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, Toscano MA, Bianco GA, Kobelt P, Handjiski B, Tirado I, Markert UR, Klapp BF, Poirier F, Szekeres-Bartho J, Rabinovich GA, Arck PC. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13:1450–1457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

