Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle
- PMID: 20053633
- PMCID: PMC2816184
- DOI: 10.1242/jcs.042234
Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle
Abstract
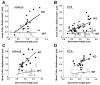
A prominent feature of striated muscle is the regular lateral alignment of adjacent sarcomeres. An important intermyofibrillar linking protein is the intermediate filament protein desmin, and based on biochemical and structural studies in primary cultures of myocytes it has been proposed that desmin interacts with the sarcomeric protein nebulin. Here we tested whether nebulin is part of a novel biomechanical linker complex, by using a recently developed nebulin knockout (KO) mouse model and measuring Z-disk displacement in adjacent myofibrils of both extensor digitorum longus (EDL) and soleus muscle. Z-disk displacement increased as sarcomere length (SL) was increased and the increase was significantly larger in KO fibers than in wild-type (WT) fibers; results in 3-day-old and 10-day-old mice were similar. Immunoelectron microscopy revealed reduced levels of desmin in intermyofibrillar spaces adjacent to Z-disks in KO fibers compared with WT fibers. We also performed siRNA knockdown of nebulin and expressed modules within the Z-disk portion of nebulin (M160-M170) in quail myotubes and found that this prevented the mature Z-disk localization of desmin filaments. Combined, these data suggest a model in which desmin attaches to the Z-disk through an interaction with nebulin. Finally, because nebulin has been proposed to play a role in specifying Z-disk width, we also measured Z-disk width in nebulin KO mice. Results show that most Z-disks of KO mice were modestly increased in width (approximately 80 nm in soleus and approximately 40 nm in EDL fibers) whereas a small subset had severely increased widths (up to approximately 1 microm) and resembled nemaline rod bodies. In summary, structural studies on a nebulin KO mouse show that in the absence of nebulin, Z-disks are significantly wider and that myofibrils are misaligned. Thus the functional roles of nebulin extend beyond thin filament length regulation and include roles in maintaining physiological Z-disk widths and myofibrillar connectivity.
Figures

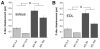





Similar articles
-
Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo.EMBO J. 2006 Aug 23;25(16):3843-55. doi: 10.1038/sj.emboj.7601242. Epub 2006 Aug 10. EMBO J. 2006. PMID: 16902413 Free PMC article.
-
Expressing a Z-disk nebulin fragment in nebulin-deficient mouse muscle: effects on muscle structure and function.Skelet Muscle. 2020 Jan 28;10(1):2. doi: 10.1186/s13395-019-0219-9. Skelet Muscle. 2020. PMID: 31992366 Free PMC article.
-
Evidence for increased myofibrillar mobility in desmin-null mouse skeletal muscle.J Exp Biol. 2002 Feb;205(Pt 3):321-5. doi: 10.1242/jeb.205.3.321. J Exp Biol. 2002. PMID: 11854369
-
New insights into the structural roles of nebulin in skeletal muscle.J Biomed Biotechnol. 2010;2010:968139. doi: 10.1155/2010/968139. Epub 2010 Jun 1. J Biomed Biotechnol. 2010. PMID: 20589077 Free PMC article. Review.
-
Obscurin: a multitasking muscle giant.J Muscle Res Cell Motil. 2005;26(6-8):419-26. doi: 10.1007/s10974-005-9024-7. J Muscle Res Cell Motil. 2005. PMID: 16625317 Review.
Cited by
-
Nebulin binding impedes mutant desmin filament assembly.Mol Biol Cell. 2013 Jun;24(12):1918-32. doi: 10.1091/mbc.E12-11-0840. Epub 2013 Apr 24. Mol Biol Cell. 2013. PMID: 23615443 Free PMC article.
-
Deleting nebulin's C-terminus reveals its importance to sarcomeric structure and function and is sufficient to invoke nemaline myopathy.Hum Mol Genet. 2019 May 15;28(10):1709-1725. doi: 10.1093/hmg/ddz016. Hum Mol Genet. 2019. PMID: 30689900 Free PMC article.
-
Nebulin interactions with actin and tropomyosin are altered by disease-causing mutations.Skelet Muscle. 2014 Aug 1;4:15. doi: 10.1186/2044-5040-4-15. eCollection 2014. Skelet Muscle. 2014. PMID: 25110572 Free PMC article.
-
Triggering typical nemaline myopathy with compound heterozygous nebulin mutations reveals myofilament structural changes as pathomechanism.Nat Commun. 2020 Jun 1;11(1):2699. doi: 10.1038/s41467-020-16526-9. Nat Commun. 2020. PMID: 32483185 Free PMC article.
-
Nebulin: big protein with big responsibilities.J Muscle Res Cell Motil. 2020 Mar;41(1):103-124. doi: 10.1007/s10974-019-09565-3. Epub 2020 Jan 25. J Muscle Res Cell Motil. 2020. PMID: 31982973 Free PMC article. Review.
References
-
- Almenar-Queralt A., Gregorio C. C., Fowler V. M. (1999). Tropomodulin assembles early in myofibrillogenesis in chick skeletal muscle: evidence that thin filaments rearrange to form striated myofibrils. J. Cell Sci. 112, 1111-1123 - PubMed
-
- Bang M. L., Gregorio C., Labeit S. (2002). Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 137, 119-127 - PubMed
-
- Capetanaki Y., Bloch R. J., Kouloumenta A., Mavroidis M., Psarras S. (2007). Muscle intermediate filaments and their links to membranes and membranous organelles. Exp. Cell Res. 313, 2063-2076 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

