Cdc20 is required for the post-anaphase, KEN-dependent degradation of centromere protein F
- PMID: 20053638
- PMCID: PMC2816182
- DOI: 10.1242/jcs.062075
Cdc20 is required for the post-anaphase, KEN-dependent degradation of centromere protein F
Abstract
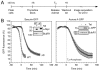
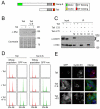
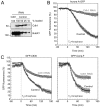
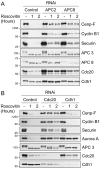
Progression through mitosis and cytokinesis requires the sequential proteolysis of several cell-cycle regulators. This proteolysis is mediated by the ubiquitin-proteasome system, with the E3 ligase being the anaphase-promoting complex, also known as the cyclosome (APC/C). The APC/C is regulated by two activators, namely Cdc20 and Cdh1. The current view is that prior to anaphase, the APC/C is activated by Cdc20, but that following anaphase, APC/C switches to Cdh1-dependent activation. However, here we present an analysis of the kinetochore protein Cenp-F that is inconsistent with this notion. Although it has long been appreciated that Cenp-F is degraded sometime during or after mitosis, exactly when and how has not been clear. Here we show that degradation of Cenp-F initiates about six minutes after anaphase, and that this is dependent on a C-terminal KEN-box. Although these two observations are consistent with Cenp-F being a substrate of Cdh1-activated APC/C, Cenp-F is degraded normally in Cdh1-null cells. By contrast, RNAi-mediated repression of APC/C subunits or Cdc20 does inhibit Cenp-F degradation. These findings therefore suggest that the APC/C does not simply 'switch' upon anaphase onset; rather, our observations indicate that Cdc20 also contributes to post-anaphase activation of the APC/C. We also show that the post-anaphase, KEN-box-dependent degradation of Cenp-F requires it to be farnesylated, a post-translational modification usually linked to membrane association. Because so many of the behaviours of Cenp-F are farnesylation-dependent, we suggest that this modification plays a more global role in Cenp-F function.
Figures



References
-
- Ameri K., Harris A. L. (2008). Activating transcription factor 4. Int. J. Biochem. Cell Biol. 40, 14-21 - PubMed
-
- Ashar H. R., James L., Gray K., Carr D., Black S., Armstrong L., Bishop W. R., Kirschmeier P. (2000). Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J. Biol. Chem. 275, 30451-30457 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

