Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity
- PMID: 20053639
- PMCID: PMC2816183
- DOI: 10.1242/jcs.039180
Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity
Abstract
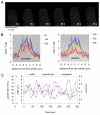
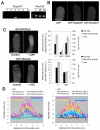
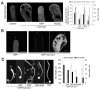
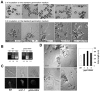
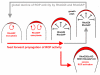
Rapid tip growth allows for efficient development of highly elongated cells (e.g. neuronal axons, fungal hyphae and pollen tubes) and requires an elaborate spatiotemporal regulation of the growing region. Here, we use the pollen tube as a model to investigate the mechanism regulating the growing region. ROPs (Rho-related GTPases from plants) are essential for pollen tip growth and display oscillatory activity changes in the apical plasma membrane (PM). By manipulating the ROP activity level, we showed that the PM distribution of ROP activity as an apical cap determines the tip growth region and that efficient tip growth requires an optimum level of the apical ROP1 activity. Excessive ROP activation induced the enlargement of the tip growth region, causing growth depolarization and reduced tube elongation. Time-lapse analysis suggests that the apical ROP1 cap is generated by lateral propagation of a localized ROP activity. Subcellular localization and gain- and loss-of-function analyses suggest that RhoGDI- and RhoGAP-mediated global inhibition limits the lateral propagation of apical ROP1 activity. We propose that the balance between the lateral propagation and the global inhibition maintains an optimal apical ROP1 cap and generates the apical ROP1 activity oscillation required for efficient pollen-tube elongation.
Figures



Similar articles
-
Rapid tip growth: insights from pollen tubes.Semin Cell Dev Biol. 2011 Oct;22(8):816-24. doi: 10.1016/j.semcdb.2011.06.004. Epub 2011 Jun 25. Semin Cell Dev Biol. 2011. PMID: 21729760 Free PMC article. Review.
-
A tip-localized RhoGAP controls cell polarity by globally inhibiting Rho GTPase at the cell apex.Curr Biol. 2008 Dec 23;18(24):1907-16. doi: 10.1016/j.cub.2008.11.057. Curr Biol. 2008. PMID: 19108776 Free PMC article.
-
ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin.J Exp Bot. 2003 Jan;54(380):93-101. doi: 10.1093/jxb/erg035. J Exp Bot. 2003. PMID: 12456759 Review.
-
Glycolysis regulates pollen tube polarity via Rho GTPase signaling.PLoS Genet. 2018 Apr 27;14(4):e1007373. doi: 10.1371/journal.pgen.1007373. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29702701 Free PMC article.
-
A genome-wide analysis of Arabidopsis Rop-interactive CRIB motif-containing proteins that act as Rop GTPase targets.Plant Cell. 2001 Dec;13(12):2841-56. doi: 10.1105/tpc.010218. Plant Cell. 2001. PMID: 11752391 Free PMC article.
Cited by
-
Rapid tip growth: insights from pollen tubes.Semin Cell Dev Biol. 2011 Oct;22(8):816-24. doi: 10.1016/j.semcdb.2011.06.004. Epub 2011 Jun 25. Semin Cell Dev Biol. 2011. PMID: 21729760 Free PMC article. Review.
-
A Rho-based reaction-diffusion system governs cell wall patterning in metaxylem vessels.Sci Rep. 2018 Aug 1;8(1):11542. doi: 10.1038/s41598-018-29543-y. Sci Rep. 2018. PMID: 30069009 Free PMC article.
-
Overexpression of the tomato pollen receptor kinase LePRK1 rewires pollen tube growth to a blebbing mode.Plant Cell. 2014 Sep;26(9):3538-55. doi: 10.1105/tpc.114.127381. Epub 2014 Sep 5. Plant Cell. 2014. PMID: 25194029 Free PMC article.
-
2, 6-Dichlorobenzonitrile causes multiple effects on pollen tube growth beyond altering cellulose synthesis in Pinus bungeana Zucc.PLoS One. 2013 Oct 11;8(10):e76660. doi: 10.1371/journal.pone.0076660. eCollection 2013. PLoS One. 2013. PMID: 24146903 Free PMC article.
-
The Microtubule-Associated Protein MAP18 Affects ROP2 GTPase Activity during Root Hair Growth.Plant Physiol. 2017 May;174(1):202-222. doi: 10.1104/pp.16.01243. Epub 2017 Mar 17. Plant Physiol. 2017. PMID: 28314794 Free PMC article.
References
-
- Alonso J. M., Stepanova A. N., Leisse T. J., Kim C. J., Chen H., Shinn P., Stevenson D. K., Zimmerman J., Barajas P., Cheuk R., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653-657 - PubMed
-
- Bischoff F., Vahlkamp L., Molendijk A., Palme K. (2000). Localization of AtROP4 and AtROP6 and interaction with the guanine nucleotide dissociation inhibitor AtRhoGDI1 from Arabidopsis. Plant Mol. Biol. 42, 515-530 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

