Gain-of-function mutation in FGFR3 in mice leads to decreased bone mass by affecting both osteoblastogenesis and osteoclastogenesis
- PMID: 20053668
- PMCID: PMC3115638
- DOI: 10.1093/hmg/ddp590
Gain-of-function mutation in FGFR3 in mice leads to decreased bone mass by affecting both osteoblastogenesis and osteoclastogenesis
Abstract
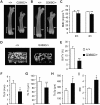
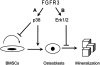
Achondroplasia (ACH) is a short-limbed dwarfism resulting from gain-of-function mutations in fibroblast growth factor receptor 3 (FGFR3). Previous studies have shown that ACH patients have impaired chondrogenesis, but the effects of FGFR3 on bone formation and bone remodeling at adult stages of ACH have not been fully investigated. Using micro-computed tomography and histomorphometric analyses, we found that 2-month-old Fgfr3(G369C/+) mice (mouse model mimicking human ACH) showed decreased bone mass due to reduced trabecular bone volume and bone mineral density, defect in bone mineralization and increased osteoclast numbers and activity. Compared with primary cultures of bone marrow stromal cells (BMSCs) from wild-type mice, Fgfr3(G369C/+) cultures showed decreased cell proliferation, increased osteogenic differentiation including up-regulation of alkaline phosphatase activity and expressions of osteoblast marker genes, and reduced bone matrix mineralization. Furthermore, our studies also suggest that decreased cell proliferation and enhanced osteogenic differentiation observed in Fgfr3(G369C/+) BMSCs are caused by up-regulation of p38 phosphorylation and that enhanced Erk1/2 activity is responsible for the impaired bone matrix mineralization. In addition, in vitro osteoclast formation and bone resorption assays demonstrated that osteoclast numbers and bone resorption area were increased in cultured bone marrow cells derived from Fgfr3(G369C/+) mice. These findings demonstrate that gain-of-function mutation in FGFR3 leads to decreased bone mass by regulating both osteoblast and osteoclast activities. Our studies provide new insight into the mechanism underlying the development of ACH.
Figures

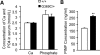


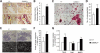
Similar articles
-
PTH 1-34 Ameliorates the Osteopenia and Delayed Healing of Stabilized Tibia Fracture in Mice with Achondroplasia Resulting from Gain-Of-Function Mutation of FGFR3.Int J Biol Sci. 2017 Sep 21;13(10):1254-1265. doi: 10.7150/ijbs.21258. eCollection 2017. Int J Biol Sci. 2017. PMID: 29104492 Free PMC article.
-
Activated FGFR3 promotes bone formation via accelerating endochondral ossification in mouse model of distraction osteogenesis.Bone. 2017 Dec;105:42-49. doi: 10.1016/j.bone.2017.05.016. Epub 2017 Aug 10. Bone. 2017. PMID: 28802681
-
Gly369Cys mutation in mouse FGFR3 causes achondroplasia by affecting both chondrogenesis and osteogenesis.J Clin Invest. 1999 Dec;104(11):1517-25. doi: 10.1172/JCI6690. J Clin Invest. 1999. PMID: 10587515 Free PMC article.
-
Achondroplasia: from genotype to phenotype.Joint Bone Spine. 2008 Mar;75(2):125-30. doi: 10.1016/j.jbspin.2007.06.007. Epub 2007 Sep 25. Joint Bone Spine. 2008. PMID: 17950653 Review.
-
C-Type Natriuretic Peptide Analog as Therapy for Achondroplasia.Endocr Dev. 2016;30:98-105. doi: 10.1159/000439334. Epub 2015 Dec 10. Endocr Dev. 2016. PMID: 26684019 Review.
Cited by
-
Whole-body and segmental analysis of body composition in adult males with achondroplasia using dual X-ray absorptiometry.PLoS One. 2019 Mar 19;14(3):e0213806. doi: 10.1371/journal.pone.0213806. eCollection 2019. PLoS One. 2019. PMID: 30889196 Free PMC article.
-
FGFR2 mutation confers a less drastic gain of function in mesenchymal stem cells than in fibroblasts.Stem Cell Rev Rep. 2012 Sep;8(3):685-95. doi: 10.1007/s12015-011-9327-6. Stem Cell Rev Rep. 2012. PMID: 22048896 Free PMC article.
-
A Ser252Trp mutation in fibroblast growth factor receptor 2 (FGFR2) mimicking human Apert syndrome reveals an essential role for FGF signaling in the regulation of endochondral bone formation.PLoS One. 2014 Jan 28;9(1):e87311. doi: 10.1371/journal.pone.0087311. eCollection 2014. PLoS One. 2014. PMID: 24489893 Free PMC article.
-
PTH 1-34 Ameliorates the Osteopenia and Delayed Healing of Stabilized Tibia Fracture in Mice with Achondroplasia Resulting from Gain-Of-Function Mutation of FGFR3.Int J Biol Sci. 2017 Sep 21;13(10):1254-1265. doi: 10.7150/ijbs.21258. eCollection 2017. Int J Biol Sci. 2017. PMID: 29104492 Free PMC article.
-
An Improved Immunostaining and Imaging Methodology to Determine Cell and Protein Distributions within the Bone Environment.J Histochem Cytochem. 2016 Mar;64(3):168-78. doi: 10.1369/0022155415626765. Epub 2015 Dec 30. J Histochem Cytochem. 2016. PMID: 26718242 Free PMC article.
References
-
- Ornitz D.M., Marie P.J. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev. 2002;16:1446–1465. - PubMed
-
- Su N., Du X., Chen L. FGF signaling: its role in bone development and human skeleton diseases. Front. Biosci. 2008;13:2842–2865. - PubMed
-
- Naski M.C., Ornitz D.M. FGF signaling in skeletal development. Front. Biosci. 1998;3:d781–d794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

