Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite
- PMID: 20053676
- PMCID: PMC2828963
- DOI: 10.1091/mbc.e09-11-0967
Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite
Abstract
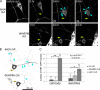
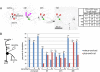
Axon regeneration is crucial for recovery after trauma to the nervous system. For neurons to recover from complete axon removal they must respecify a dendrite as an axon: a complete reversal of polarity. We show that Drosophila neurons in vivo can convert a dendrite to a regenerating axon and that this process involves rebuilding the entire neuronal microtubule cytoskeleton. Two major microtubule rearrangements are specifically induced by axon and not dendrite removal: 1) 10-fold up-regulation of the number of growing microtubules and 2) microtubule polarity reversal. After one dendrite reverses its microtubules, it initiates tip growth and takes on morphological and molecular characteristics of an axon. Only neurons with a single dendrite that reverses polarity are able to initiate tip growth, and normal microtubule plus-end dynamics are required to initiate this growth. In addition, we find that JNK signaling is required for both the up-regulation of microtubule dynamics and microtubule polarity reversal initiated by axon injury. We conclude that regulation of microtubule dynamics and polarity in response to JNK signaling is key to initiating regeneration of an axon from a dendrite.
Figures





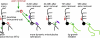
Similar articles
-
Neurons survive simultaneous injury to axons and dendrites and regrow both types of processes in vivo.Dev Biol. 2020 Sep 15;465(2):108-118. doi: 10.1016/j.ydbio.2020.07.006. Epub 2020 Jul 18. Dev Biol. 2020. PMID: 32687893 Free PMC article.
-
The microtubule-severing protein fidgetin acts after dendrite injury to promote their degeneration.J Cell Sci. 2016 Sep 1;129(17):3274-81. doi: 10.1242/jcs.188540. Epub 2016 Jul 13. J Cell Sci. 2016. PMID: 27411367 Free PMC article.
-
Directed microtubule growth, +TIPs, and kinesin-2 are required for uniform microtubule polarity in dendrites.Curr Biol. 2010 Dec 21;20(24):2169-77. doi: 10.1016/j.cub.2010.11.050. Epub 2010 Dec 9. Curr Biol. 2010. PMID: 21145742 Free PMC article.
-
Principles of microtubule polarity in linear cells.Dev Biol. 2022 Mar;483:112-117. doi: 10.1016/j.ydbio.2022.01.004. Epub 2022 Jan 8. Dev Biol. 2022. PMID: 35016908 Free PMC article. Review.
-
Polarity Sorting of Microtubules in the Axon.Trends Neurosci. 2018 Feb;41(2):77-88. doi: 10.1016/j.tins.2017.11.002. Epub 2017 Nov 30. Trends Neurosci. 2018. PMID: 29198454 Free PMC article. Review.
Cited by
-
A novel injury paradigm in the central nervous system of adult Drosophila: molecular, cellular and functional aspects.Dis Model Mech. 2021 May 1;14(5):dmm044669. doi: 10.1242/dmm.044669. Epub 2021 Jun 1. Dis Model Mech. 2021. PMID: 34061177 Free PMC article.
-
Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology.J Cell Biol. 2011 May 16;193(4):769-84. doi: 10.1083/jcb.201008050. Epub 2011 May 9. J Cell Biol. 2011. PMID: 21555464 Free PMC article.
-
Patronin governs minus-end-out orientation of dendritic microtubules to promote dendrite pruning in Drosophila.Elife. 2019 Mar 28;8:e39964. doi: 10.7554/eLife.39964. Elife. 2019. PMID: 30920370 Free PMC article.
-
Intrinsic mechanisms for axon regeneration: insights from injured axons in Drosophila.Curr Opin Genet Dev. 2017 Jun;44:84-91. doi: 10.1016/j.gde.2017.01.009. Epub 2017 Feb 21. Curr Opin Genet Dev. 2017. PMID: 28232273 Free PMC article. Review.
-
To nucleate or not, that is the question in neurons.Neurosci Lett. 2021 Apr 23;751:135806. doi: 10.1016/j.neulet.2021.135806. Epub 2021 Mar 8. Neurosci Lett. 2021. PMID: 33705928 Free PMC article. Review.
References
-
- Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y., Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 1999;19:2322–2329. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

