NPP-16/Nup50 function and CDK-1 inactivation are associated with anoxia-induced prophase arrest in Caenorhabditis elegans
- PMID: 20053678
- PMCID: PMC2828959
- DOI: 10.1091/mbc.e09-09-0787
NPP-16/Nup50 function and CDK-1 inactivation are associated with anoxia-induced prophase arrest in Caenorhabditis elegans
Abstract
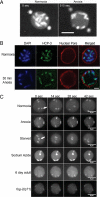
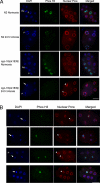
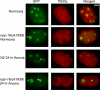
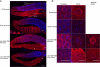
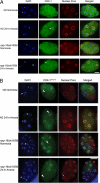
Oxygen, an essential nutrient, is sensed by a multiple of cellular pathways that facilitate the responses to and survival of oxygen deprivation. The Caenorhabditis elegans embryo exposed to severe oxygen deprivation (anoxia) enters a state of suspended animation in which cell cycle progression reversibly arrests at specific stages. The mechanisms regulating interphase, prophase, or metaphase arrest in response to anoxia are not completely understood. Characteristics of arrested prophase blastomeres and oocytes are the alignment of condensed chromosomes at the nuclear periphery and an arrest of nuclear envelope breakdown. Notably, anoxia-induced prophase arrest is suppressed in mutant embryos lacking nucleoporin NPP-16/NUP50 function, indicating that this nucleoporin plays an important role in prophase arrest in wild-type embryos. Although the inactive form of cyclin-dependent kinase (CDK-1) is detected in wild-type-arrested prophase blastomeres, the inactive state is not detected in the anoxia exposed npp-16 mutant. Furthermore, we found that CDK-1 localizes near chromosomes in anoxia-exposed embryos. These data support the notion that NPP-16 and CDK-1 function to arrest prophase blastomeres in C. elegans embryos. The anoxia-induced shift of cells from an actively dividing state to an arrested state reveals a previously uncharacterized prophase checkpoint in the C. elegans embryo.
Figures

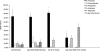

Similar articles
-
Characterization of sub-nuclear changes in Caenorhabditis elegans embryos exposed to brief, intermediate and long-term anoxia to analyze anoxia-induced cell cycle arrest.BMC Cell Biol. 2005 Dec 20;6:47. doi: 10.1186/1471-2121-6-47. BMC Cell Biol. 2005. PMID: 16368008 Free PMC article.
-
Dephosphorylation of cell cycle-regulated proteins correlates with anoxia-induced suspended animation in Caenorhabditis elegans.Mol Biol Cell. 2002 May;13(5):1473-83. doi: 10.1091/mbc.01-12-0594. Mol Biol Cell. 2002. PMID: 12006646 Free PMC article.
-
Cell cycle control by daf-21/Hsp90 at the first meiotic prophase/metaphase boundary during oogenesis in Caenorhabditis elegans.Dev Growth Differ. 2006 Jan;48(1):25-32. doi: 10.1111/j.1440-169X.2006.00841.x. Dev Growth Differ. 2006. PMID: 16466390
-
Suspended animation, diapause and quiescence: arresting the cell cycle in C. elegans.Cell Cycle. 2012 May 1;11(9):1672-9. doi: 10.4161/cc.19444. Epub 2012 May 1. Cell Cycle. 2012. PMID: 22510566 Free PMC article. Review.
-
Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells.Mol Hum Reprod. 2010 Sep;16(9):654-64. doi: 10.1093/molehr/gaq034. Epub 2010 May 7. Mol Hum Reprod. 2010. PMID: 20453035 Free PMC article. Review.
Cited by
-
TMEM160 Promotes Tumor Growth in Lung Adenocarcinoma and Cervical Adenocarcinoma Cell Lines.Int J Mol Sci. 2025 Jan 27;26(3):1097. doi: 10.3390/ijms26031097. Int J Mol Sci. 2025. PMID: 39940865 Free PMC article.
-
Dissection of the NUP107 nuclear pore subcomplex reveals a novel interaction with spindle assembly checkpoint protein MAD1 in Caenorhabditis elegans.Mol Biol Cell. 2012 Mar;23(5):930-44. doi: 10.1091/mbc.E11-11-0927. Epub 2012 Jan 11. Mol Biol Cell. 2012. PMID: 22238360 Free PMC article.
-
Use of time lapse microscopy to visualize anoxia-induced suspended animation in C. elegans embryos.J Vis Exp. 2012 Dec 3;(70):e4319. doi: 10.3791/4319. J Vis Exp. 2012. PMID: 23242305 Free PMC article.
-
Cell Biology of the Caenorhabditis elegans Nucleus.Genetics. 2017 Jan;205(1):25-59. doi: 10.1534/genetics.116.197160. Genetics. 2017. PMID: 28049702 Free PMC article. Review.
-
Predicting Human Protein Subcellular Locations by Using a Combination of Network and Function Features.Front Genet. 2021 Nov 5;12:783128. doi: 10.3389/fgene.2021.783128. eCollection 2021. Front Genet. 2021. PMID: 34804131 Free PMC article.
References
-
- Akhtar A., Gasser S. M. The nuclear envelope and transcriptional control. Nat. Rev. Genet. 2007;8:507–517. - PubMed
-
- Angelo G., Van Gilst M. R. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. - PubMed
-
- Beaudouin J., Gerlich D., Daigle N., Eils R., Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

