The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone
- PMID: 20053716
- PMCID: PMC2817608
- DOI: 10.1210/me.2009-0354
The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone
Abstract
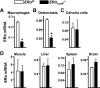
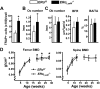
Estrogens attenuate osteoclastogenesis and stimulate osteoclast apoptosis, but the molecular mechanism and contribution of these effects to the overall antiosteoporotic efficacy of estrogens remain controversial. We selectively deleted the estrogen receptor (ER)alpha from the monocyte/macrophage cell lineage in mice (ERalpha(LysM)(-/-)) and found a 2-fold increase in osteoclast progenitors in the marrow and the number of osteoclasts in cancellous bone, along with a decrease in cancellous bone mass. After loss of estrogens these mice failed to exhibit the expected increase in osteoclast progenitors, the number of osteoclasts in bone, and further loss of cancellous bone. However, they lost cortical bone indistinguishably from their littermate controls. Mature osteoclasts from ERalpha(LysM)(-/-) were resistant to the proapoptotic effect of 17beta-estradiol. Nonetheless, the effects of estrogens on osteoclasts were unhindered in mice bearing an ERalpha knock-in mutation that prevented binding to DNA. Moreover, a polymeric form of estrogen that is not capable of stimulating the nuclear-initiated actions of ERalpha was as effective as 17beta-estradiol in inducing osteoclast apoptosis in cells with the wild-type ERalpha. We conclude that estrogens attenuate osteoclast generation and life span via cell autonomous effects mediated by DNA-binding-independent actions of ERalpha. Elimination of these effects is sufficient for loss of bone in the cancellous compartment in which complete perforation of trabeculae by osteoclastic resorption precludes subsequent refilling of the cavities by the bone-forming osteoblasts. However, additional effects of estrogens on osteoblasts, osteocytes, and perhaps other cell types are required for their protective effects on the cortical compartment, which constitutes 80% of the skeleton.
Figures

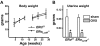
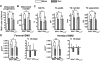


Similar articles
-
The Effects of Androgens on Murine Cortical Bone Do Not Require AR or ERα Signaling in Osteoblasts and Osteoclasts.J Bone Miner Res. 2015 Jul;30(7):1138-49. doi: 10.1002/jbmr.2485. J Bone Miner Res. 2015. PMID: 25704845 Free PMC article.
-
Role of ERαMISS in the Effect of Estradiol on Cancellous and Cortical Femoral Bone in Growing Female Mice.Endocrinology. 2016 Jun;157(6):2533-44. doi: 10.1210/en.2015-1994. Epub 2016 Apr 22. Endocrinology. 2016. PMID: 27105385
-
Estrogens maintain bone mass by regulating expression of genes controlling function and life span in mature osteoclasts.Ann N Y Acad Sci. 2009 Sep;1173 Suppl 1:E31-9. doi: 10.1111/j.1749-6632.2009.04954.x. Ann N Y Acad Sci. 2009. PMID: 19751412
-
Sex steroids and bone.Recent Prog Horm Res. 2002;57:385-409. doi: 10.1210/rp.57.1.385. Recent Prog Horm Res. 2002. PMID: 12017554 Review.
-
Androgens and bone.Endocr Rev. 2004 Jun;25(3):389-425. doi: 10.1210/er.2003-0003. Endocr Rev. 2004. PMID: 15180950 Review.
Cited by
-
SERMs have substance-specific effects on bone, and these effects are mediated via ERαAF-1 in female mice.Am J Physiol Endocrinol Metab. 2016 Jun 1;310(11):E912-8. doi: 10.1152/ajpendo.00488.2015. Epub 2016 Apr 5. Am J Physiol Endocrinol Metab. 2016. PMID: 27048997 Free PMC article.
-
Down-regulation of Irf8 by Lyz2-cre/loxP accelerates osteoclast differentiation in vitro.Cytotechnology. 2017 Jun;69(3):443-450. doi: 10.1007/s10616-016-0013-z. Epub 2016 Aug 8. Cytotechnology. 2017. PMID: 27502007 Free PMC article.
-
The RANKL distal control region is required for the increase in RANKL expression, but not the bone loss, associated with hyperparathyroidism or lactation in adult mice.Mol Endocrinol. 2012 Feb;26(2):341-8. doi: 10.1210/me.2011-1149. Epub 2011 Dec 29. Mol Endocrinol. 2012. PMID: 22207718 Free PMC article.
-
Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice.Aging Cell. 2019 Jun;18(3):e12923. doi: 10.1111/acel.12923. Epub 2019 Feb 17. Aging Cell. 2019. PMID: 30773784 Free PMC article.
-
FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation.Nat Commun. 2014 Apr 30;5:3773. doi: 10.1038/ncomms4773. Nat Commun. 2014. PMID: 24781012 Free PMC article.
References
-
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 - PubMed
-
- Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS, Manolagas SC 2008 Apoptosis and bone cells. In: Bilezikian JP, Raisz LG, Martin T, eds. Principles of bone biology. San Diego: Academic Press; 235–259
-
- Manolagas SC 2006 Perspective: choreography from the tomb: an emerging role of dying osteocytes in the purposeful, and perhaps not so purposeful, targeting of bone remodeling. BoneKey-Osteovision 3:5–14; 10.1138/20060193
-
- Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T 2006 Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res 21:605–615 - PubMed
-
- Verborgt O, Gibson GJ, Schaffler MB 2000 Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 15:60–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

