A conserved lysine in the thyroid hormone receptor-alpha1 DNA-binding domain, mutated in hepatocellular carcinoma, serves as a sensor for transcriptional regulation
- PMID: 20053725
- PMCID: PMC2808454
- DOI: 10.1158/1541-7786.MCR-09-0425
A conserved lysine in the thyroid hormone receptor-alpha1 DNA-binding domain, mutated in hepatocellular carcinoma, serves as a sensor for transcriptional regulation
Abstract
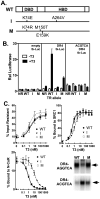
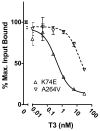
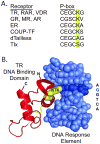
Nuclear receptors are hormone-regulated transcription factors that play key roles in normal physiology and development; conversely, mutant nuclear receptors are associated with a wide variety of neoplastic and endocrine disorders. Typically, these receptor mutants function as dominant negatives and can interfere with wild-type receptor activity. Dominant-negative thyroid hormone receptor (TR) mutations have been identified in over 60% of the human hepatocellular carcinomas analyzed. Most of these mutant TRs are defective for corepressor release or coactivator binding in vitro, accounting for their transcriptional defects in vivo. However, two HCC-TR mutants that function as dominant-negative receptors in cells display near-normal properties in vitro, raising questions about the molecular basis behind their transcriptional defects. We report here that a single amino acid substitution, located at the same position in the DNA-binding domain of both mutants, is responsible for their impaired transcriptional activation and dominant-negative properties. Significantly, this amino acid, K74 in TRalpha, is highly conserved in all known nuclear receptors and seems to function as an allosteric sensor that regulates the transcriptional activity of these receptors in response to binding to their DNA recognition sequences. We provide evidence that these two human hepatocellular carcinoma mutants have acquired dominant-negative function as a result of disruption of this allosteric sensing. Our results suggest a novel mechanism by which nuclear receptors can acquire transcriptional defects and contribute to neoplastic disease.
Figures


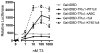
Similar articles
-
Thyroid hormone receptors mutated in liver cancer function as distorted antimorphs.Oncogene. 2006 Jun 15;25(25):3576-88. doi: 10.1038/sj.onc.1209389. Epub 2006 Jan 23. Oncogene. 2006. PMID: 16434963 Free PMC article.
-
An F-domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha.Gen Comp Endocrinol. 2008 Jan 1;155(1):176-89. doi: 10.1016/j.ygcen.2007.04.012. Epub 2007 Apr 27. Gen Comp Endocrinol. 2008. PMID: 17583703 Free PMC article.
-
Thyroid hormone receptor mutants implicated in human hepatocellular carcinoma display an altered target gene repertoire.Oncogene. 2009 Nov 26;28(47):4162-74. doi: 10.1038/onc.2009.265. Epub 2009 Sep 14. Oncogene. 2009. PMID: 19749797 Free PMC article.
-
Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance.Trends Endocrinol Metab. 2005 May-Jun;16(4):176-82. doi: 10.1016/j.tem.2005.03.008. Trends Endocrinol Metab. 2005. PMID: 15860414 Review.
-
Integrative analysis of aberrant Wnt signaling in hepatitis B virus-related hepatocellular carcinoma.World J Gastroenterol. 2015 May 28;21(20):6317-28. doi: 10.3748/wjg.v21.i20.6317. World J Gastroenterol. 2015. PMID: 26034368 Free PMC article. Review.
Cited by
-
Thyroid hormone receptor localization in target tissues.J Endocrinol. 2018 Apr;237(1):R19-R34. doi: 10.1530/JOE-17-0708. Epub 2018 Feb 12. J Endocrinol. 2018. PMID: 29440347 Free PMC article. Review.
-
Mechanisms of thyroid hormone action.J Clin Invest. 2012 Sep;122(9):3035-43. doi: 10.1172/JCI60047. Epub 2012 Sep 4. J Clin Invest. 2012. PMID: 22945636 Free PMC article. Review.
-
Recruitment of the oncoprotein v-ErbA to aggresomes.Mol Cell Endocrinol. 2011 Jan 30;332(1-2):196-212. doi: 10.1016/j.mce.2010.10.012. Epub 2010 Nov 12. Mol Cell Endocrinol. 2011. PMID: 21075170 Free PMC article.
-
Non-genomic Actions of Thyroid Hormones Regulate the Growth and Angiogenesis of T Cell Lymphomas.Front Endocrinol (Lausanne). 2019 Feb 13;10:63. doi: 10.3389/fendo.2019.00063. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30814977 Free PMC article. Review.
-
Mislocalization of Cancer-associated Thyroid Hormone Receptor Mutants.Nucl Receptor Res. 2020;2020:https://web.archive.org/web/20210227193123/https://www.kenzpub.com/journals/nurr/inpress/2020/101453/. Nucl Receptor Res. 2020. PMID: 35280700 Free PMC article.
References
-
- Harvey CB, Williams GR. Mechanism of thyroid hormone action. Thyroid. 2002;12(6):441–6. - PubMed
-
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81(3):1097–142. - PubMed
-
- Flamant F, Baxter JD, Forrest D, et al. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev. 2006;58(4):705–11. - PubMed
-
- Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu Rev Physiol. 2004;66:315–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

