Involvement of ceramide in the propagation of Japanese encephalitis virus
- PMID: 20053738
- PMCID: PMC2826033
- DOI: 10.1128/JVI.02499-09
Involvement of ceramide in the propagation of Japanese encephalitis virus
Abstract
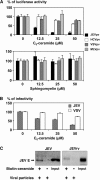
Japanese encephalitis virus (JEV) is a mosquito-borne RNA virus and one of the most important flaviviruses in the medical and veterinary fields. Although cholesterol has been shown to participate in both the entry and replication steps of JEV, the mechanisms of infection, including the cellular receptors of JEV, remain largely unknown. To clarify the infection mechanisms of JEV, we generated pseudotype (JEVpv) and recombinant (JEVrv) vesicular stomatitis viruses bearing the JEV envelope protein. Both JEVpv and JEVrv exhibited high infectivity for the target cells, and JEVrv was able to propagate and form foci as did authentic JEV. Anti-JEV envelope antibodies neutralized infection of the viruses. Treatment of cells with inhibitors for vacuolar ATPase and clathrin-mediated endocytosis reduced the infectivity of JEVpv, suggesting that JEVpv enters cells via pH- and clathrin-dependent endocytic pathways. Although treatment of the particles of JEVpv, JEVrv, and JEV with cholesterol drastically reduced the infectivity as previously reported, depletion of cholesterol from the particles by treatment with methyl beta-cyclodextrin enhanced infectivity. Furthermore, treatment of cells with sphingomyelinase (SMase), which hydrolyzes membrane-bound sphingomyelin to ceramide, drastically enhanced infection with JEVpv and propagation of JEVrv, and these enhancements were inhibited by treatment with an SMase inhibitor or C(6)-ceramide. These results suggest that ceramide plays crucial roles in not only entry but also egress processes of JEV, and they should assist in the clarification of JEV propagation and the development of novel therapeutics against diseases caused by infection with flaviviruses.
Figures





References
-
- Aizaki, H., K. Morikawa, M. Fukasawa, H. Hara, Y. Inoue, H. Tani, K. Saito, M. Nishijima, K. Hanada, Y. Matsuura, M. M. Lai, T. Miyamura, T. Wakita, and T. Suzuki. 2008. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol. 82:5715-5724. - PMC - PubMed
-
- Bollinger, C. R., V. Teichgraber, and E. Gulbins. 2005. Ceramide-enriched membrane domains. Biochim. Biophys. Acta 1746:284-294. - PubMed
-
- Boonsanay, V., and D. R. Smith. 2007. Entry into and production of the Japanese encephalitis virus from C6/36 cells. Intervirology 50:85-92. - PubMed
-
- Chatterjee, S. 1993. Neutral sphingomyelinase increases the binding, internalization, and degradation of low density lipoproteins and synthesis of cholesteryl ester in cultured human fibroblasts. J. Biol. Chem. 268:3401-3406. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

