Characterization of a single-cycle rabies virus-based vaccine vector
- PMID: 20053743
- PMCID: PMC2826042
- DOI: 10.1128/JVI.01870-09
Characterization of a single-cycle rabies virus-based vaccine vector
Abstract
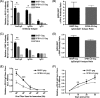
Recombinant rabies virus (RV)-based vectors have demonstrated their efficacy in generating long-term, antigen-specific immune responses in murine and monkey models. However, replication-competent viral vectors pose significant safety concerns due to vector pathogenicity. RV pathogenicity is largely attributed to its glycoprotein (RV-G), which facilitates the attachment and entry of RV into host cells. We have developed a live, single-cycle RV by deletion of the G gene from an RV vaccine vector expressing HIV-1 Gag (SPBN-DeltaG-Gag). Passage of SPBN-DeltaG-Gag on cells stably expressing RV-G allowed efficient propagation of the G-deleted RV. The in vivo immunogenicity data comparing single-cycle RV to a replication-competent control (BNSP-Gag) showed lower RV-specific antibodies; however, the overall isotype profiles (IgG2a/IgG1) were similar for the two vaccine vectors. Despite this difference, mice immunized with SPBN-DeltaG-Gag and BNSP-Gag mounted similar levels of Gag-specific CD8(+) T-cell responses as measured by major histocompatibility complex class I Gag-tetramer staining, gamma interferon-enzyme-linked immunospot assay, and cytotoxic T-cell assay. Moreover, these cellular responses were maintained equally at immunization titers as low as 10(3) focus-forming units for both RV vaccine vectors. CD8(+) T-cell responses were significantly enhanced by a boost with a single-cycle RV complemented with a heterologous vesicular stomatitis virus glycoprotein. These findings demonstrate that single-cycle RV is an effective alternative to replication-competent RV vectors for future development of vaccines for HIV-1 and other infectious diseases.
Figures
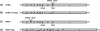



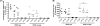


Similar articles
-
Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene.Vaccine. 2008 Nov 25;26(50):6405-14. doi: 10.1016/j.vaccine.2008.08.069. Epub 2008 Sep 18. Vaccine. 2008. PMID: 18804506 Free PMC article.
-
Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector.J Virol. 2001 Sep;75(18):8724-32. doi: 10.1128/jvi.75.18.8724-8732.2001. J Virol. 2001. PMID: 11507217 Free PMC article.
-
Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic.J Virol. 2003 Jan;77(1):237-44. doi: 10.1128/jvi.77.1.237-244.2003. J Virol. 2003. PMID: 12477829 Free PMC article.
-
Canine adenovirus based rabies vaccines.Dev Biol (Basel). 2008;131:467-76. Dev Biol (Basel). 2008. PMID: 18634509 Review.
-
Experimental rabies vaccines for humans.Expert Rev Vaccines. 2010 Oct;9(10):1177-86. doi: 10.1586/erv.10.105. Expert Rev Vaccines. 2010. PMID: 20923268 Free PMC article. Review.
Cited by
-
An anterograde rabies virus vector for high-resolution large-scale reconstruction of 3D neuron morphology.Brain Struct Funct. 2015;220(3):1369-79. doi: 10.1007/s00429-014-0730-z. Epub 2014 Apr 11. Brain Struct Funct. 2015. PMID: 24723034 Free PMC article.
-
Rhabdovirus-based vaccine platforms against henipaviruses.J Virol. 2015 Jan;89(1):144-54. doi: 10.1128/JVI.02308-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320306 Free PMC article.
-
Induction of CD8 T cell heterologous protection by a single dose of single-cycle infectious influenza virus.J Virol. 2014 Oct;88(20):12006-16. doi: 10.1128/JVI.01847-14. Epub 2014 Aug 6. J Virol. 2014. PMID: 25100831 Free PMC article.
-
Dendritic cells infected by recombinant rabies virus vaccine vector expressing HIV-1 Gag are immunogenic even in the presence of vector-specific immunity.Vaccine. 2010 Dec 10;29(1):130-40. doi: 10.1016/j.vaccine.2010.08.042. Epub 2010 Aug 20. Vaccine. 2010. PMID: 20728525 Free PMC article.
-
Controlled viral glycoprotein expression as a safety feature in a bivalent rabies-ebola vaccine.Virus Res. 2015 Feb 2;197:54-8. doi: 10.1016/j.virusres.2014.11.028. Epub 2014 Dec 4. Virus Res. 2015. PMID: 25481284 Free PMC article.
References
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. - PubMed
-
- Bozac, A., E. Berto, F. Vasquez, P. Grandi, A. Caputo, R. Manservigi, B. Ensoli, and P. Marconi. 2006. Expression of human immunodeficiency virus type 1 tat from a replication-deficient herpes simplex type 1 vector induces antigen-specific T cell responses. Vaccine 24:7148-7158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

