Functional analysis of RNA structures present at the 3' extremity of the murine norovirus genome: the variable polypyrimidine tract plays a role in viral virulence
- PMID: 20053745
- PMCID: PMC2826041
- DOI: 10.1128/JVI.02053-09
Functional analysis of RNA structures present at the 3' extremity of the murine norovirus genome: the variable polypyrimidine tract plays a role in viral virulence
Erratum in
- J Virol. 2010 Oct;84(20):10943. Reese, Jivan [corrected to Rees, Jivan]
Abstract
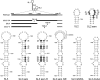
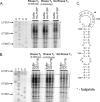
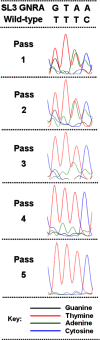
Interactions of host cell factors with RNA sequences and structures in the genomes of positive-strand RNA viruses play various roles in the life cycles of these viruses. Our understanding of the functional RNA elements present in norovirus genomes to date has been limited largely to in vitro analysis. However, we recently used reverse genetics to identify evolutionarily conserved RNA structures and sequences required for norovirus replication. We have now undertaken a more detailed analysis of RNA structures present at the 3' extremity of the murine norovirus (MNV) genome. Biochemical data indicate the presence of three stable stem-loops, including two in the untranslated region, and a single-stranded polypyrimidine tract [p(Y)] of variable length between MNV isolates, within the terminal stem-loop structure. The well-characterized host cell pyrimidine binding proteins PTB and PCBP bound the 3'-untranslated region via an interaction with this variable sequence. Viruses lacking the p(Y) tract were viable both in cell culture and upon mouse infection, demonstrating that this interaction was not essential for virus replication. However, competition analysis with wild-type MNV in cell culture indicated that the loss of the p(Y) tract was associated with a fitness cost. Furthermore, a p(Y)-deleted mutant showed a reduction in virulence in the STAT1(-/-) mouse model, highlighting the role of RNA structures in norovirus pathogenesis. This work highlights how, like with other positive-strand RNA viruses, RNA structures present at the termini of the norovirus genome play important roles in virus replication and virulence.
Figures

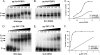


References
-
- Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
-
- Chang, K.-O., S. V. Sosnovtsev, G. Belliot, A. D. King, and K. Y. Green. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463-473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

