Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model
- PMID: 20053748
- PMCID: PMC2826051
- DOI: 10.1128/JVI.01805-09
Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model
Abstract
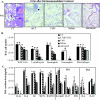
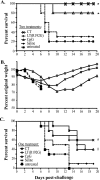
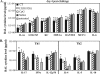
Prophylactic or therapeutic immunomodulation is an antigen-independent strategy that induces nonspecific immune system activation, thereby enhancing host defense to disease. In this study, we investigated the effect of prophylactic immunomodulation on the outcome of influenza virus infection using three bacterially derived immune-enhancing agents known for promoting distinct immunological profiles. BALB/c mice were treated nasally with either cholera toxin (CT), a mutant form of the CT-related Escherichia coli heat-labile enterotoxin designated LT(R192G), or CpG oligodeoxynucleotide. Mice were subsequently challenged with a lethal dose of influenza A/PR/8/34 virus 24 h after the last immunomodulation treatment and either monitored for survival or sacrificed postchallenge for viral and immunological analysis. Treatment with the three immunomodulators prevented or delayed mortality and weight loss, but only CT and LT(R192G) significantly reduced initial lung viral loads as measured by plaque assay. Analysis performed 4 days postinfection indicated that prophylactic treatments with CT, LT(R192G), or CpG resulted in significantly increased numbers of CD4 T cells, B cells, and dendritic cells and altered costimulatory marker expression in the airways of infected mice, coinciding with reduced expression of pulmonary chemokines and the appearance of inducible bronchus-associated lymphoid tissue-like structures in the lungs. Collectively, these results suggest that, despite different immunomodulatory mechanisms, CT, LT(R192G), and CpG induce an initial inflammatory process and enhance the immune response to primary influenza virus challenge while preventing potentially damaging chemokine expression. These studies provide insight into the immunological parameters and immune modulation strategies that have the potential to enhance the nonspecific host response to influenza virus infection.
Figures



References
-
- Anonymous. 2008. Influenza fact sheet no. 211. World Health Organization, Geneva, Switzerland.
-
- Bromander, A., J. Holmgren, and N. Lycke. 1991. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J. Immunol. 146:2908-2914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

