Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs
- PMID: 20053749
- PMCID: PMC2838122
- DOI: 10.1128/JVI.02252-09
Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs
Abstract
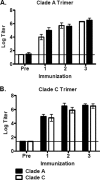
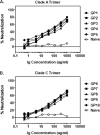
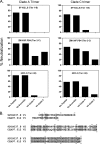
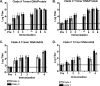
The native envelope (Env) spike on the surface of human immunodeficiency virus type 1 (HIV-1) is trimeric, and thus trimeric Env vaccine immunogens are currently being explored in preclinical immunogenicity studies. Key challenges have included the production and purification of biochemically homogeneous and stable trimers and the evaluation of these immunogens utilizing standardized virus panels for neutralization assays. Here we report the binding and neutralizing antibody (NAb) responses elicited by clade A (92UG037.8) and clade C (CZA97.012) Env gp140 trimer immunogens in guinea pigs. These trimers have been selected and engineered for optimal biochemical stability and have defined antigenic properties. Purified gp140 trimers with Ribi adjuvant elicited potent, cross-clade NAb responses against tier 1 viruses as well as detectable but low-titer NAb responses against select tier 2 viruses from clades A, B, and C. In particular, the clade C trimer elicited NAbs that neutralized 27%, 20%, and 47% of tier 2 viruses from clades A, B, and C, respectively. Heterologous DNA prime, protein boost as well as DNA prime, recombinant adenovirus boost regimens expressing these antigens, however, did not result in an increased magnitude or breadth of NAb responses in this system. These data demonstrate the immunogenicity of stable, homogeneous clade A and clade C gp140 trimers and exemplify the utility of standardized tier 1 and tier 2 virus panels for assessing the NAb responses of candidate HIV-1 Env immunogens.
Figures
References
-
- Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. - PMC - PubMed
-
- Beddows, S., M. Franti, A. K. Dey, M. Kirschner, S. P. Iyer, D. C. Fisch, T. Ketas, E. Yuste, R. C. Desrosiers, P. J. Klasse, P. J. Maddon, W. C. Olson, and J. P. Moore. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329-340. - PubMed
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Berman, P. W., D. J. Eastman, D. M. Wilkes, G. R. Nakamura, T. J. Gregory, D. Schwartz, G. Gorse, R. Belshe, M. L. Clements, and R. A. Byrn. 1994. Comparison of the immune response to recombinant gp120 in humans and chimpanzees. AIDS 8:591-601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

