No increase in hepatitis B virus (HBV)-specific CD8+ T cells in patients with HIV-1-HBV coinfections following HBV-active highly active antiretroviral therapy
- PMID: 20053751
- PMCID: PMC2826062
- DOI: 10.1128/JVI.02124-09
No increase in hepatitis B virus (HBV)-specific CD8+ T cells in patients with HIV-1-HBV coinfections following HBV-active highly active antiretroviral therapy
Abstract
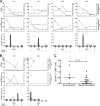
Following treatment of hepatitis B virus (HBV) monoinfection, HBV-specific T-cell responses increase significantly; however, little is known about the recovery of HBV-specific T-cell responses following HBV-active highly active antiretroviral therapy (HAART) in HIV-HBV coinfected patients. HIV-HBV coinfected patients who were treatment naïve and initiating HBV-active HAART were recruited as part of a prospective cohort study in Thailand and followed for 48 weeks (n = 24). Production of gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha) in both HBV- and HIV-specific CD8(+) T cells was quantified using intracellular cytokine staining on whole blood. Following HBV-active HAART, the median (interquartile range) log decline from week 0 to week 48 for HBV DNA was 5.8 log (range, 3.4 to 6.7) IU/ml, and for HIV RNA it was 3.1 (range, 2.9 to 3.5) log copies/ml (P < 0.001 for both). The frequency of HIV Gag-specific CD8(+) T-cell responses significantly decreased (IFN-gamma, P < 0.001; TNF-alpha, P = 0.05). In contrast, there was no significant change in the frequency (IFN-gamma, P = 0.21; TNF-alpha, P = 0.61; and IFN-gamma and TNF-alpha, P = 0.11) or magnitude (IFN-gamma, P = 0.13; TNF-alpha, P = 0.13; and IFN-gamma and TNF-alpha, P = 0.13) of HBV-specific CD8(+) T-cell responses over 48 weeks of HBV-active HAART. Of the 14 individuals who were HBV e antigen (HBeAg) positive, 5/14 (36%) lost HBeAg during the 48 weeks of follow-up. HBV-specific CD8(+) T cells were detected in 4/5 (80%) of patients prior to HBeAg loss. Results from this study show no sustained change in the HBV-specific CD8(+) T-cell response following HBV-active HAART. These findings may have implications for the duration of treatment of HBV in HIV-HBV coinfected patients, particularly in HBeAg-positive disease.
Figures


References
-
- Alatrakchi, N., C. Duvivier, D. Costagliola, A. Samri, A. G. Marcelin, G. Kamkamidze, M. Astriti, R. Agher, V. Calvez, B. Autran, and C. Katlama. 2005. Persistent low viral load on antiretroviral therapy is associated with T cell-mediated control of HIV replication. AIDS 19:25-33. - PubMed
-
- Appay, V., D. C. Douek, and D. A. Price. 2008. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 14:623-628. - PubMed
-
- Bedossa, P., and T. Poynard. 1996. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289-293. - PubMed
-
- Boni, C., A. Penna, A. Bertoletti, V. Lamonaca, I. Rapti, G. Missale, M. Pilli, S. Urbani, A. Cavalli, S. Cerioni, R. Panebianco, J. Jenkins, and C. Ferrari. 2003. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J. Hepatol. 39:595-605. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

