Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program
- PMID: 20053767
- PMCID: PMC3003872
- DOI: 10.1158/1535-7163.MCT-09-0952
Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program
Abstract
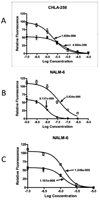
Rapamycin demonstrated broad-spectrum tumor growth inhibition activity against the in vivo panels of childhood tumors used in the Pediatric Preclinical Testing Program (PPTP). Here we have evaluated rapamycin combined with agents used frequently in the treatment of childhood malignancies. Rapamycin was tested in vitro against 23 cell lines alone or in combination with melphalan, cisplatin, vincristine, or dexamethasone (leukemic models only). In vivo, the impact of combining rapamycin with a cytotoxic agent was evaluated using two measures: 1) the therapeutic enhancement measure, and 2) a linear regression model for time-to-event to formally evaluate for sub- and supraadditivity for the combination compared to the agents used alone. Combining rapamycin with cytotoxic agents in vitro gave predominantly subadditive or additive effects, except for dexamethasone in leukemia models for which supra-additive activity was observed. In vivo testing demonstrated that therapeutic enhancement was common for rapamycin in combination with cyclophosphamide and occurred for 4 of 11 evaluable xenografts for the rapamycin and vincristine combination. The combinations of rapamycin with either cyclophosphamide or vincristine were significantly more effective than the respective standard agents used alone at their maximum tolerated doses (MTD) for most evaluable xenografts. The combination of rapamycin and cisplatin produced excessive toxicity requiring cisplatin dose reductions, and therapeutic enhancement was not observed for this combination. Addition of rapamycin to either cyclophosphamide or vincristine at their respective MTDs appears promising, as these combinations are relatively well tolerated and as many of the pediatric preclinical models evaluated demonstrated therapeutic enhancement for these combinations.
Figures

Similar articles
-
Combination testing of cediranib (AZD2171) against childhood cancer models by the pediatric preclinical testing program.Pediatr Blood Cancer. 2012 Apr;58(4):566-71. doi: 10.1002/pbc.23159. Epub 2011 Apr 29. Pediatr Blood Cancer. 2012. PMID: 21538824 Free PMC article.
-
Evaluation of patritumab with or without erlotinib in combination with standard cytotoxic agents against pediatric sarcoma xenograft models.Pediatr Blood Cancer. 2018 Feb;65(2):10.1002/pbc.26870. doi: 10.1002/pbc.26870. Epub 2017 Oct 28. Pediatr Blood Cancer. 2018. PMID: 29080385 Free PMC article.
-
mTOR inhibition sensitizes gastric cancer to alkylating chemotherapy in vivo.Anticancer Res. 2008 Nov-Dec;28(6A):3801-8. Anticancer Res. 2008. PMID: 19189667
-
Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program.Pediatr Blood Cancer. 2008 Apr;50(4):799-805. doi: 10.1002/pbc.21296. Pediatr Blood Cancer. 2008. PMID: 17635004
-
The pediatric preclinical testing program: description of models and early testing results.Pediatr Blood Cancer. 2007 Dec;49(7):928-40. doi: 10.1002/pbc.21078. Pediatr Blood Cancer. 2007. PMID: 17066459
Cited by
-
Preclinical In Vivo Modeling of Pediatric Sarcoma-Promises and Limitations.J Clin Med. 2021 Apr 8;10(8):1578. doi: 10.3390/jcm10081578. J Clin Med. 2021. PMID: 33918045 Free PMC article. Review.
-
A review of targeted therapies evaluated by the pediatric preclinical testing program for osteosarcoma.Front Oncol. 2013 May 31;3:132. doi: 10.3389/fonc.2013.00132. eCollection 2013. Front Oncol. 2013. PMID: 23755370 Free PMC article.
-
Effectiveness of metronomic chemotherapy in a child with medulloblastoma: A case report.Oncol Lett. 2023 Mar 30;25(5):194. doi: 10.3892/ol.2023.13780. eCollection 2023 May. Oncol Lett. 2023. PMID: 37113402 Free PMC article.
-
Inhibition of SAPK2/p38 enhances sensitivity to mTORC1 inhibition by blocking IRES-mediated translation initiation in glioblastoma.Mol Cancer Ther. 2011 Dec;10(12):2244-56. doi: 10.1158/1535-7163.MCT-11-0478. Epub 2011 Sep 12. Mol Cancer Ther. 2011. PMID: 21911485 Free PMC article.
-
Initial testing (stage 1) of the phosphatidylinositol 3' kinase inhibitor, SAR245408 (XL147) by the pediatric preclinical testing program.Pediatr Blood Cancer. 2013 May;60(5):791-8. doi: 10.1002/pbc.24301. Epub 2012 Sep 21. Pediatr Blood Cancer. 2013. PMID: 23002019 Free PMC article.
References
-
- Dilling MB, Dias P, Shapiro DN, Germain GS, Johnson RK, Houghton PJ. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer research. 1994;54(4):903–907. - PubMed
-
- Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(4):799–805. - PubMed
-
- Houchens DP, Ovejera AA, Riblet SM, Slagel DE. Human brain tumor xenografts in nude mice as a chemotherapy model. European journal of cancer & clinical oncology. 1983;19(6):799–805. - PubMed
-
- Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. - PubMed
-
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356(22):2271–2281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

