Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program
- PMID: 20053767
- PMCID: PMC3003872
- DOI: 10.1158/1535-7163.MCT-09-0952
Stage 2 combination testing of rapamycin with cytotoxic agents by the Pediatric Preclinical Testing Program
Abstract
Rapamycin demonstrated broad-spectrum tumor growth inhibition activity against the in vivo panels of childhood tumors used in the Pediatric Preclinical Testing Program (PPTP). Here we have evaluated rapamycin combined with agents used frequently in the treatment of childhood malignancies. Rapamycin was tested in vitro against 23 cell lines alone or in combination with melphalan, cisplatin, vincristine, or dexamethasone (leukemic models only). In vivo, the impact of combining rapamycin with a cytotoxic agent was evaluated using two measures: 1) the therapeutic enhancement measure, and 2) a linear regression model for time-to-event to formally evaluate for sub- and supraadditivity for the combination compared to the agents used alone. Combining rapamycin with cytotoxic agents in vitro gave predominantly subadditive or additive effects, except for dexamethasone in leukemia models for which supra-additive activity was observed. In vivo testing demonstrated that therapeutic enhancement was common for rapamycin in combination with cyclophosphamide and occurred for 4 of 11 evaluable xenografts for the rapamycin and vincristine combination. The combinations of rapamycin with either cyclophosphamide or vincristine were significantly more effective than the respective standard agents used alone at their maximum tolerated doses (MTD) for most evaluable xenografts. The combination of rapamycin and cisplatin produced excessive toxicity requiring cisplatin dose reductions, and therapeutic enhancement was not observed for this combination. Addition of rapamycin to either cyclophosphamide or vincristine at their respective MTDs appears promising, as these combinations are relatively well tolerated and as many of the pediatric preclinical models evaluated demonstrated therapeutic enhancement for these combinations.
Figures
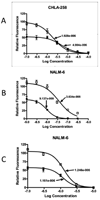

References
-
- Dilling MB, Dias P, Shapiro DN, Germain GS, Johnson RK, Houghton PJ. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer research. 1994;54(4):903–907. - PubMed
-
- Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2008;50(4):799–805. - PubMed
-
- Houchens DP, Ovejera AA, Riblet SM, Slagel DE. Human brain tumor xenografts in nude mice as a chemotherapy model. European journal of cancer & clinical oncology. 1983;19(6):799–805. - PubMed
-
- Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–135. - PubMed
-
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England journal of medicine. 2007;356(22):2271–2281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

