A pilot randomized study of smokeless tobacco use among smokers not interested in quitting: changes in smoking behavior and readiness to quit
- PMID: 20053788
- PMCID: PMC2816197
- DOI: 10.1093/ntr/ntp186
A pilot randomized study of smokeless tobacco use among smokers not interested in quitting: changes in smoking behavior and readiness to quit
Abstract
Introduction: Several prior studies suggest that smokeless tobacco use results in less carcinogenic risk than does cigarette smoking. Whether smokers will use smokeless tobacco is unclear, as is the impact of such use on long-term smoking behavior and cessation. It is equally plausible that smokeless tobacco use among smokers could either (a) increase total tobacco exposure and undermine motivation to quit or (b) decrease overall tobacco exposure, motivate smokers to quit, and enhance cessation. Either outcome is of major public health significance.
Methods: In this small (N = 31), short-term (2 week) pilot study, smokers uninterested in quitting were randomized to (a) receive Ariva or Stonewall (both spitless and smokeless tobacco lozenges) or (b) continue smoking conventional cigarettes.
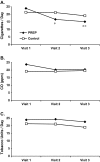
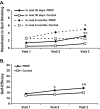
Results: Ariva/Stonewall use led to a significant reduction (40%, 95% CI: 24%-55%) in cigarettes per day, no significant increases in total tobacco use (cigarettes + Ariva/Stonewall; p > .05), and significant increases in two measures of readiness to quit, either in the next month (p < .001) or within the next 6 months (p = .04), as well as significant increases in self-efficacy to quit smoking (p < .001). No such changes were found among smokers maintained on conventional cigarettes.
Discussion: These results suggest no deleterious effect on short-term smoking and quitting behavior among smokers who use smokeless tobacco. More broadly, this study suggests a strong need for a large prospective randomized clinical trial to more accurately assess the long-term viability of smokeless tobacco use as a method for cessation induction among unmotivated smokers.
Figures
Similar articles
-
Brief, instructional smokeless tobacco use among cigarette smokers who do not intend to quit: a pilot randomized clinical trial.Nicotine Tob Res. 2014 Apr;16(4):397-405. doi: 10.1093/ntr/ntt161. Epub 2013 Oct 15. Nicotine Tob Res. 2014. PMID: 24130144 Free PMC article. Clinical Trial.
-
Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study.Cancer Epidemiol Biomarkers Prev. 2011 Jan;20(1):91-100. doi: 10.1158/1055-9965.EPI-10-0349. Epub 2010 Nov 10. Cancer Epidemiol Biomarkers Prev. 2011. PMID: 21068204 Free PMC article. Clinical Trial.
-
An Avatar-Led Intervention Promotes Smoking Cessation in Young Adults: A Pilot Randomized Clinical Trial.Ann Behav Med. 2020 Oct 1;54(10):747-760. doi: 10.1093/abm/kaaa013. Ann Behav Med. 2020. PMID: 32383736 Clinical Trial.
-
[Smoking reduction and temporary abstinence: new approaches for smoking cessation].J Mal Vasc. 2003 Dec;28(5):293-300. J Mal Vasc. 2003. PMID: 14978435 Review. French.
-
[Extreme solutions for those who do not succeed to quit smoking. About smokeless tobacco and harm reduction (part II)].Pneumologia. 2008 Jul-Sep;57(3):178-81. Pneumologia. 2008. PMID: 18998333 Review. Romanian.
Cited by
-
US smokers' reactions to a brief trial of oral nicotine products.Harm Reduct J. 2011 Jan 10;8:1. doi: 10.1186/1477-7517-8-1. Harm Reduct J. 2011. PMID: 21219609 Free PMC article.
-
Effect of Smoking Reduction Therapy on Smoking Cessation for Smokers without an Intention to Quit: An Updated Systematic Review and Meta-Analysis of Randomized Controlled.Int J Environ Res Public Health. 2015 Aug 25;12(9):10235-53. doi: 10.3390/ijerph120910235. Int J Environ Res Public Health. 2015. PMID: 26308034 Free PMC article.
-
The scientific foundation for tobacco harm reduction, 2006-2011.Harm Reduct J. 2011 Jul 29;8:19. doi: 10.1186/1477-7517-8-19. Harm Reduct J. 2011. PMID: 21801389 Free PMC article.
-
A fresh look at tobacco harm reduction: the case for the electronic cigarette.Harm Reduct J. 2013 Oct 4;10:19. doi: 10.1186/1477-7517-10-19. Harm Reduct J. 2013. PMID: 24090432 Free PMC article. Review.
-
The acceptability of nicotine containing products as alternatives to cigarettes: findings from two pilot studies.Harm Reduct J. 2011 Oct 12;8:27. doi: 10.1186/1477-7517-8-27. Harm Reduct J. 2011. PMID: 21992707 Free PMC article.
References
-
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. - PubMed
-
- Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of Consulting and Clinical Psychology. 2004;72:371–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

