Melphalan, prednisone, thalidomide and defibrotide in relapsed/refractory multiple myeloma: results of a multicenter phase I/II trial
- PMID: 20053869
- PMCID: PMC2895039
- DOI: 10.3324/haematol.2009.017913
Melphalan, prednisone, thalidomide and defibrotide in relapsed/refractory multiple myeloma: results of a multicenter phase I/II trial
Abstract
Background: Defibrotide is a novel orally bioavailable polydisperse oligonucleotide with anti-thrombotic and anti-adhesive effects. In SCID/NOD mice, defibrotide showed activity in human myeloma xenografts. This phase I/II study was conducted to identify the most appropriate dose of defibrotide in combination with melphalan, prednisone and thalidomide in patients with relapsed and relapsed/refractory multiple myeloma, and to determine its safety and tolerability as part of this regimen.
Design and methods: This was a phase I/II, multicenter, dose-escalating, non-comparative, open label study. Oral melphalan was administered at a dose of 0.25 mg/kg on days 1-4, prednisone at a dose of 1.5 mg/kg also on days 1-4 and thalidomide at a dose of 50-100 mg/day continuously. Defibrotide was administered orally at three dose-levels: 2.4, 4.8 or 7.2 g on days 1-4 and 1.6, 3.2, or 4.8 g on days 5-35.
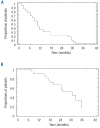
Results: Twenty-four patients with relapsed/refractory multiple myeloma were enrolled. No dose-limiting toxicity was observed. In all patients, the complete response plus very good partial response rate was 9%, and the partial response rate was 43%. The 1-year progression-free survival and 1-year overall survival rates were 34% and 90%, respectively. The most frequent grade 3-4 adverse events included neutropenia, thrombocytopenia, anemia and fatigue. Deep vein thrombosis was reported in only one patient.
Conclusions: This combination of melphalan, prednisone and thalidomide together with defibrotide showed anti-tumor activity with a favorable tolerability. The maximum tolerated dose of defibrotide was identified as 7.2 g p.o. on days 1-4 followed by 4.8 g p.o. on days 5-35. Further trials are needed to confirm the role of this regimen and to evaluate the combination of defibrotide with new drugs.
Trial registration: ClinicalTrials.gov NCT00406978.
Figures
Similar articles
-
Lenalidomide, melphalan, prednisone and thalidomide (RMPT) for relapsed/refractory multiple myeloma.Leukemia. 2010 May;24(5):1037-42. doi: 10.1038/leu.2010.58. Epub 2010 Apr 8. Leukemia. 2010. PMID: 20376079 Clinical Trial.
-
Phase II study of melphalan, thalidomide and prednisone combined with oral panobinostat in patients with relapsed/refractory multiple myeloma.Leuk Lymphoma. 2012 Sep;53(9):1722-7. doi: 10.3109/10428194.2012.664844. Epub 2012 Mar 16. Leuk Lymphoma. 2012. PMID: 22335534 Clinical Trial.
-
Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial.J Clin Oncol. 2009 Aug 1;27(22):3664-70. doi: 10.1200/JCO.2008.21.0948. Epub 2009 May 18. J Clin Oncol. 2009. PMID: 19451428 Clinical Trial.
-
Update on the use of defibrotide.Expert Opin Biol Ther. 2012 Mar;12(3):353-61. doi: 10.1517/14712598.2012.657623. Epub 2012 Jan 28. Expert Opin Biol Ther. 2012. PMID: 22283742 Review.
-
Management of the relapsed/refractory myeloma patient: strategies incorporating lenalidomide.Semin Hematol. 2005 Oct;42(4 Suppl 4):S9-15. doi: 10.1053/j.seminhematol.2005.10.004. Semin Hematol. 2005. PMID: 16344100 Review.
Cited by
-
Endothelial stress products and coagulation markers in patients with multiple myeloma treated with lenalidomide plus dexamethasone: an observational study.Br J Haematol. 2013 Feb;160(3):351-8. doi: 10.1111/bjh.12152. Epub 2012 Dec 13. Br J Haematol. 2013. PMID: 23240658 Free PMC article.
-
A Focus on Relapsed Multiple Myeloma.J Adv Pract Oncol. 2022 Jul;13(Suppl 4):15-21. doi: 10.6004/jadpro.2022.13.5.11. Epub 2022 Jul 28. J Adv Pract Oncol. 2022. PMID: 35937465 Free PMC article.
-
The potential of heparanase as a therapeutic target in cancer.Biochem Pharmacol. 2014 May 1;89(1):12-9. doi: 10.1016/j.bcp.2014.02.010. Epub 2014 Feb 22. Biochem Pharmacol. 2014. PMID: 24565907 Free PMC article.
-
Very late antigen-4 (α(4)β(1) Integrin) targeted PET imaging of multiple myeloma.PLoS One. 2013;8(2):e55841. doi: 10.1371/journal.pone.0055841. Epub 2013 Feb 8. PLoS One. 2013. PMID: 23409060 Free PMC article.
-
Treatment of relapsed and refractory multiple myeloma.Haematologica. 2016 Apr;101(4):396-406. doi: 10.3324/haematol.2015.129189. Haematologica. 2016. PMID: 27033237 Free PMC article. Review.
References
-
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: cancer incidence, mortality and prelevance worldwide. Lyon. France: GLOBOCAN; 2004.
-
- Cavo M, Benni M, Ronconi S, Fiacchini M, Gozzetti A, Zamagni E, et al. Melphalanprednisone versus alternating combination VAD/MP or VND/MP as primary therapy for multiple myeloma: Final analysis of a randomized clinical study. Haematologica. 2002;87(9):934–42. - PubMed
-
- Myeloma Trialists’ Collaborative Group. Combination chemotherapy versus melphalan and prednisone as treatment of multiple myeloma: An overview of 6,633 patients from 27 randomized trials. J Clin Oncol. 1998;16(12):3832–42. - PubMed
-
- Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–14. - PubMed
-
- uldbrandsen N, Waage A, Gismin P, Turesson I, Juliusson G, Abildgaard N, et al. A randomised placebo controlled study with melphalan/prednisone vs melphalan/prednisone/thalidomide: quality of life and toxicity [abstract] Haematologica. 2008;93:0209.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical