Immunogenicity of a live recombinant Salmonella enterica serovar typhimurium vaccine expressing pspA in neonates and infant mice born from naive and immunized mothers
- PMID: 20053873
- PMCID: PMC2837964
- DOI: 10.1128/CVI.00413-09
Immunogenicity of a live recombinant Salmonella enterica serovar typhimurium vaccine expressing pspA in neonates and infant mice born from naive and immunized mothers
Abstract
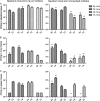
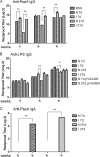
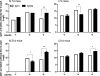
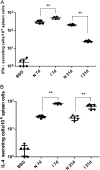
We are developing a Salmonella vectored vaccine to prevent infant pneumonia and other diseases caused by Streptococcus pneumoniae. One prerequisite for achieving this goal is to construct and evaluate new recombinant attenuated Salmonella vaccine (RASV) strains suitable for use in neonates and infants. Salmonella enterica serovar Typhimurium strain chi9558(pYA4088) specifies delivery of the pneumococcal protective antigen PspA and can protect adult mice from challenge with S. pneumoniae. This strain is completely safe for oral delivery to day-old and infant mice. Here we assess the colonizing ability, immunogenicity, and protective efficacy of chi9558(pYA4088) in neonatal mice. Colonization was assessed in mice 0, 2, 4, or 7 days of age after oral inoculation. In the presence of maternal antibodies, the colonization of lymphoid tissues was delayed, but the immune responses were enhanced in mice born to immunized mothers. Both oral and intranasal routes were used to assess immunogenicity. All orally or intranasally immunized neonatal and infant mice born to either immunized or naïve mothers developed PspA-specific mucosal and systemic immune responses. Mice born to immunized mothers produced higher titers of PspA-specific antibodies in the blood and mucosa and greater numbers of PspA-specific interleukin-4 (IL-4)-secreting cells than mice born to naïve mothers. More importantly, mice born to immune mothers showed a significant increase in protection against S. pneumoniae challenge. These results suggest that strain chi9558(pYA4088) can circumvent some of the limitations of the immature immune system in neonatal and infant mice, generating enhanced protective immune responses in the presence of maternal antibodies.
Figures
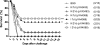
References
-
- Appelbaum, P. C. 1992. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin. Infect. Dis. 15:77-83. - PubMed
-
- Beagley, K. W., J. H. Eldridge, H. Kiyono, M. P. Everson, W. J. Koopman, T. Honjo, and J. R. McGhee. 1988. Recombinant murine IL-5 induces high rate IgA synthesis in cycling IgA-positive Peyer's patch B cells. J. Immunol. 141:2035-2042. - PubMed
-
- Black, S., H. Shinefield, R. Cohen, D. Floret, J. Gaudelus, C. Olivier, and P. Reinert. 2004. Clinical effectiveness of seven-valent pneumococcal conjugate vaccine (Prevenar) against invasive pneumococcal diseases: prospects for children in France. Arch. Pediatr. 11:843-853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

