Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex
- PMID: 20053890
- PMCID: PMC2822440
- DOI: 10.1523/JNEUROSCI.5656-08.2010
Neonatal cerebral hypoxia-ischemia impairs plasticity in rat visual cortex
Abstract
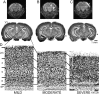
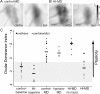
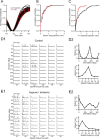
Ocular dominance plasticity (ODP) following monocular deprivation (MD) is a model of activity-dependent neural plasticity that is restricted to an early critical period regulated by maturation of inhibition. Unique developmental plasticity mechanisms may improve outcomes following early brain injury. Our objective was to determine the effects of neonatal cerebral hypoxia-ischemia (HI) on ODP. The rationale extends from observations that neonatal HI results in death of subplate neurons, a transient population known to influence development of inhibition. In rodents subjected to neonatal HI and controls, maps of visual response were derived from optical imaging during the critical period for ODP and changes in the balance of eye-specific response following MD were measured. In controls, MD results in a shift of the ocular dominance index (ODI) from a baseline of 0.15 to -0.10 (p < 0.001). Neonatal HI with moderate cortical injury impairs this shift, ODI = 0.14 (p < 0.01). Plasticity was intact in animals with mild injury and in those exposed to hypoxia alone. Neonatal HI resulted in decreased parvalbumin expression in hemispheres receiving HI compared with hypoxia alone: 23.4 versus 35.0 cells/high-power field (p = 0.01), with no change in other markers of inhibitory or excitatory neurons. Despite abnormal inhibitory neuron phenotype, spontaneous activity of single units and development of orientation selective responses were intact following neonatal HI, while overall visual responses were reduced. Our data suggest that specific plasticity mechanisms are impaired following early brain injury and that the impairment is associated with altered inhibitory neuronal development and cortical activation.
Figures




References
-
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–389. - PubMed
-
- Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. - PubMed
-
- Alpár A, Gärtner U, Härtig W, Brückner G. Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res. 2006;1120:13–22. - PubMed
-
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–1726. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
