Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window
- PMID: 20053892
- PMCID: PMC2880440
- DOI: 10.1523/JNEUROSCI.4305-09.2010
Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of I(T)window
Abstract
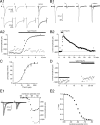
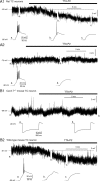
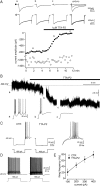
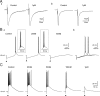
Although it is well established that low-voltage-activated T-type Ca(2+) channels play a key role in many neurophysiological functions and pathological states, the lack of selective and potent antagonists has so far hampered a detailed analysis of the full impact these channels might have on single-cell and neuronal network excitability as well as on Ca(2+) homeostasis. Recently, a novel series of piperidine-based molecules has been shown to selectively block recombinant T-type but not high-voltage-activated (HVA) Ca(2+) channels and to affect a number of physiological and pathological T-type channel-dependent behaviors. Here we directly show that one of these compounds, 3,5-dichloro-N-[1-(2,2-dimethyl-tetrahydro-pyran-4-ylmethyl)-4-fluoro-piperidin-4-ylmethyl]-benzamide (TTA-P2), exerts a specific, potent (IC(50) = 22 nm), and reversible inhibition of T-type Ca(2+) currents of thalamocortical and reticular thalamic neurons, without any action on HVA Ca(2+) currents, Na(+) currents, action potentials, and glutamatergic and GABAergic synaptic currents. Thus, under current-clamp conditions, the low-threshold Ca(2+) potential (LTCP)-dependent high-frequency burst firing of thalamic neurons is abolished by TTA-P2, whereas tonic firing remains unaltered. Using TTA-P2, we provide the first direct demonstration of the presence of a window component of Ca(2+) channels in neurons and its contribution to the resting membrane potential of thalamic neurons and to the Up state of their intrinsically generated slow (<1 Hz) oscillation. Moreover, we demonstrate that activation of only a small fraction of the T-type channel population is required to generate robust LTCPs, suggesting that LTCP-driven bursts of action potentials can be evoked at depolarized potentials where the vast majority of T-type channels are inactivated.
Figures



Similar articles
-
Inhibition of T-type calcium current in rat thalamocortical neurons by isoflurane.Neuropharmacology. 2012 Aug;63(2):266-73. doi: 10.1016/j.neuropharm.2012.03.018. Epub 2012 Apr 2. Neuropharmacology. 2012. PMID: 22491022 Free PMC article.
-
Rebound excitation triggered by synaptic inhibition in cerebellar nuclear neurons is suppressed by selective T-type calcium channel block.J Neurophysiol. 2011 Nov;106(5):2653-61. doi: 10.1152/jn.00612.2011. Epub 2011 Aug 17. J Neurophysiol. 2011. PMID: 21849607
-
TTA-P2 is a potent and selective blocker of T-type calcium channels in rat sensory neurons and a novel antinociceptive agent.Mol Pharmacol. 2011 Nov;80(5):900-10. doi: 10.1124/mol.111.073205. Epub 2011 Aug 5. Mol Pharmacol. 2011. PMID: 21821734 Free PMC article.
-
Novel neuronal and astrocytic mechanisms in thalamocortical loop dynamics.Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1675-93. doi: 10.1098/rstb.2002.1155. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626003 Free PMC article. Review.
-
The many faces of T-type calcium channels.Pflugers Arch. 2014 Mar;466(3):415-23. doi: 10.1007/s00424-013-1353-6. Epub 2013 Sep 17. Pflugers Arch. 2014. PMID: 24043572 Review.
Cited by
-
Selectivity filters and cysteine-rich extracellular loops in voltage-gated sodium, calcium, and NALCN channels.Front Physiol. 2015 May 19;6:153. doi: 10.3389/fphys.2015.00153. eCollection 2015. Front Physiol. 2015. PMID: 26042044 Free PMC article. Review.
-
Delta frequency optogenetic stimulation of the thalamic nucleus reuniens is sufficient to produce working memory deficits: relevance to schizophrenia.Biol Psychiatry. 2015 Jun 15;77(12):1098-107. doi: 10.1016/j.biopsych.2015.01.020. Epub 2015 Feb 28. Biol Psychiatry. 2015. PMID: 25891221 Free PMC article. Review.
-
L- and T-type calcium channels control aldosterone production from human adrenals.J Endocrinol. 2020 Jan;244(1):237-247. doi: 10.1530/JOE-19-0259. J Endocrinol. 2020. PMID: 31652415 Free PMC article.
-
µ-Theraphotoxin Pn3a inhibition of CaV3.3 channels reveals a novel isoform-selective drug binding site.Elife. 2022 Jul 20;11:e74040. doi: 10.7554/eLife.74040. Elife. 2022. PMID: 35858123 Free PMC article.
-
Ongoing network state controls the length of sleep spindles via inhibitory activity.Neuron. 2014 Jun 18;82(6):1367-79. doi: 10.1016/j.neuron.2014.04.046. Neuron. 2014. PMID: 24945776 Free PMC article.
References
-
- Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. - PubMed
-
- Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) Mol Pharmacol. 1995;48:540–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
