Trans-splicing-mediated improvement in a severe mouse model of spinal muscular atrophy
- PMID: 20053895
- PMCID: PMC2836862
- DOI: 10.1523/JNEUROSCI.4489-09.2010
Trans-splicing-mediated improvement in a severe mouse model of spinal muscular atrophy
Abstract
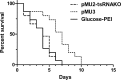
Spinal muscular atrophy is a leading genetic cause of infantile death and occurs in approximately 1/6000 live births. SMA is caused by the loss of Survival Motor Neuron-1 (SMN1), however, all patients retain at least one copy of a nearly identical gene called SMN2. While SMN2 and SMN1 are comprised of identical coding sequences, the majority of SMN2 transcripts are alternatively spliced, encoding a truncated protein that is unstable and nonfunctional. Considerable effort has focused upon modulating the SMN2 alternative splicing event since this would produce more wild-type protein. Recently we reported the development of an optimized trans-splicing system that involved the coexpression of a SMN2 trans-splicing RNA and an antisense RNA that blocks a downstream splice site in SMN2 pre-mRNA. Here, we demonstrate that in vivo delivery of the optimized trans-splicing vector increases an important SMN-dependent activity, snRNP assembly, in disease-relevant tissue in the SMA mouse model. A single injection of the vector into the intracerebral-ventricular space in SMA neonates also lessens the severity of the SMA phenotype in a severe SMA mouse model, extending survival approximately 70%. Collectively, these results provide the first in vivo demonstration that SMN2 trans-splicing leads to a lessening of the severity of the SMA phenotype and provide evidence for the power of this strategy for reprogramming genetic diseases at the pre-mRNA level.
Figures


Similar articles
-
Combination of SMN trans-splicing and a neurotrophic factor increases the life span and body mass in a severe model of spinal muscular atrophy.Hum Gene Ther. 2011 Feb;22(2):135-44. doi: 10.1089/hum.2010.114. Epub 2010 Dec 19. Hum Gene Ther. 2011. PMID: 20804424
-
Optimization of SMN trans-splicing through the analysis of SMN introns.J Mol Neurosci. 2012 Mar;46(3):459-69. doi: 10.1007/s12031-011-9614-3. Epub 2011 Aug 9. J Mol Neurosci. 2012. PMID: 21826391
-
Restoration of SMN function: delivery of a trans-splicing RNA re-directs SMN2 pre-mRNA splicing.Mol Ther. 2007 Aug;15(8):1471-8. doi: 10.1038/sj.mt.6300222. Epub 2007 Jun 5. Mol Ther. 2007. PMID: 17551501
-
Genomic Variability in the Survival Motor Neuron Genes (SMN1 and SMN2): Implications for Spinal Muscular Atrophy Phenotype and Therapeutics Development.Int J Mol Sci. 2021 Jul 23;22(15):7896. doi: 10.3390/ijms22157896. Int J Mol Sci. 2021. PMID: 34360669 Free PMC article. Review.
-
Splicing of the Survival Motor Neuron genes and implications for treatment of SMA.Front Biosci (Landmark Ed). 2010 Jun 1;15(3):1191-1204. doi: 10.2741/3670. Front Biosci (Landmark Ed). 2010. PMID: 20515750 Free PMC article. Review.
Cited by
-
Genetic therapy for the nervous system.Hum Mol Genet. 2011 Apr 15;20(R1):R28-41. doi: 10.1093/hmg/ddr110. Epub 2011 Mar 23. Hum Mol Genet. 2011. PMID: 21429918 Free PMC article. Review.
-
Pharmacology of a central nervous system delivered 2'-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates.J Pharmacol Exp Ther. 2014 Jul;350(1):46-55. doi: 10.1124/jpet.113.212407. Epub 2014 Apr 30. J Pharmacol Exp Ther. 2014. PMID: 24784568 Free PMC article.
-
Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy.J Clin Invest. 2011 Aug;121(8):3029-41. doi: 10.1172/JCI57291. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785219 Free PMC article.
-
Targeting Alternative Splicing as a Potential Therapy for Episodic Ataxia Type 2.Biomedicines. 2020 Sep 5;8(9):332. doi: 10.3390/biomedicines8090332. Biomedicines. 2020. PMID: 32899500 Free PMC article. Review.
-
Spinal Muscular Atrophy.Neurol Clin. 2015 Nov;33(4):831-46. doi: 10.1016/j.ncl.2015.07.004. Neurol Clin. 2015. PMID: 26515624 Free PMC article. Review.
References
-
- Baughan T, Shababi M, Coady TH, Dickson AM, Tullis GE, Lorson CL. Stimulating full-length SMN2 expression by delivering bifunctional RNAs via a viral vector. Mol Ther. 2006;14:54–62. - PubMed
-
- Cartegni L, Krainer AR. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet. 2002;30:377–384. - PubMed
-
- Cartegni L, Krainer AR. Correction of disease-associated exon skipping by synthetic exon-specific activators. Nat Struct Biol. 2003;10:120–125. - PubMed
-
- Coady TH, Shababi M, Tullis GE, Lorson CL. Restoration of SMN function: delivery of a trans-splicing RNA re-directs SMN2 pre-mRNA splicing. Mol Ther. 2007;15:1471–1478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
