Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors
- PMID: 20053896
- PMCID: PMC2826173
- DOI: 10.1523/JNEUROSCI.3282-09.2010
Distinct mechanisms produce functionally complementary actions of neuropeptides that are structurally related but derived from different precursors
Abstract
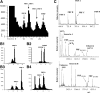
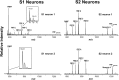
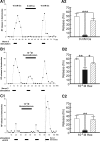
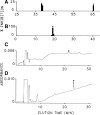
Many bioactive neuropeptides containing RFamide at their C terminus have been described in both invertebrates and vertebrates. To obtain insight into the functional logic of RFamide signaling, we investigate it here in the feeding system of Aplysia. We focus on the expression, localization, and actions of two families of RFamide peptides, the FRFamides and FMRFamide, in the central neuronal circuitry and the peripheral musculature that generate the feeding movements. We describe the cloning of the FRFamide precursor protein and show that the FRFamides and FMRFamide are derived from different precursors. We map the expression of the FRFamide and FMRFamide precursors in the feeding circuitry using in situ hybridization and immunostaining and confirm proteolytic processing of the FRFamide precursor by mass spectrometry. We show that the two precursors are expressed in different populations of sensory neurons in the feeding system. In a representative feeding muscle, we demonstrate the presence of both FRFamides and FMRFamide and their release, probably from the processes of the sensory neurons in the muscle. Both centrally and in the periphery, the FRFamides and FMRFamide act in distinct ways, apparently through distinct mechanisms, and nevertheless, from an overall functional perspective, their actions are complementary. Together, the FRFamides and FMRFamide convert feeding motor programs from ingestive to egestive and depress feeding muscle contractions. We conclude that these structurally related peptides, although derived from different precursors, expressed in different neurons, and acting through different mechanisms, remain related to each other in the functional roles that they play in the system.
Figures







Similar articles
-
Identification of a new neuropeptide precursor reveals a novel source of extrinsic modulation in the feeding system of Aplysia.J Neurosci. 2005 Oct 19;25(42):9637-48. doi: 10.1523/JNEUROSCI.2932-05.2005. J Neurosci. 2005. PMID: 16237168 Free PMC article.
-
Processing of the FMRFamide precursor protein in the snail Lymnaea stagnalis: characterization and neuronal localization of a novel peptide, 'SEEPLY'.Eur J Neurosci. 1993 Aug 1;5(8):1003-16. doi: 10.1111/j.1460-9568.1993.tb00952.x. Eur J Neurosci. 1993. PMID: 7904219
-
The enterins: a novel family of neuropeptides isolated from the enteric nervous system and CNS of Aplysia.J Neurosci. 2001 Oct 15;21(20):8247-61. doi: 10.1523/JNEUROSCI.21-20-08247.2001. J Neurosci. 2001. PMID: 11588196 Free PMC article.
-
Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides.Annu Rev Entomol. 2003;48:485-503. doi: 10.1146/annurev.ento.48.091801.112525. Epub 2002 Jun 4. Annu Rev Entomol. 2003. PMID: 12414735 Review.
-
Driving reproduction: RFamide peptides behind the wheel.Horm Behav. 2006 Dec;50(5):655-66. doi: 10.1016/j.yhbeh.2006.06.004. Epub 2006 Jul 31. Horm Behav. 2006. PMID: 16876801 Free PMC article. Review.
Cited by
-
FMRF-NH2 -related neuropeptides in Biomphalaria spp., intermediate hosts for schistosomiasis: Precursor organization and immunohistochemical localization.J Comp Neurol. 2021 Sep;529(13):3336-3358. doi: 10.1002/cne.25195. Epub 2021 Jun 11. J Comp Neurol. 2021. PMID: 34041754 Free PMC article.
-
Urotensin II in invertebrates: from structure to function in Aplysia californica.PLoS One. 2012;7(11):e48764. doi: 10.1371/journal.pone.0048764. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23144960 Free PMC article.
-
Newly Identified Aplysia SPTR-Gene Family-Derived Peptides: Localization and Function.ACS Chem Neurosci. 2018 Aug 15;9(8):2041-2053. doi: 10.1021/acschemneuro.7b00513. Epub 2018 Mar 27. ACS Chem Neurosci. 2018. PMID: 29543430 Free PMC article.
-
Neuropeptide modulation of microcircuits.Curr Opin Neurobiol. 2012 Aug;22(4):592-601. doi: 10.1016/j.conb.2012.01.003. Epub 2012 Feb 1. Curr Opin Neurobiol. 2012. PMID: 22305485 Free PMC article. Review.
-
Small-volume analysis of cell-cell signaling molecules in the brain.Neuropsychopharmacology. 2014 Jan;39(1):50-64. doi: 10.1038/npp.2013.145. Epub 2013 Jun 10. Neuropsychopharmacology. 2014. PMID: 23748227 Free PMC article. Review.
References
-
- Baxter DA, Byrne JH. Feeding behavior of Aplysia: a model system for comparing cellular mechanisms of classical and operant conditioning. Learn Mem. 2006;13:669–680. - PubMed
-
- Bechtold DA, Luckman SM. The role of RFamide peptides in feeding. J Endocrinol. 2007;192:3–15. - PubMed
-
- Brezina V. A simple technique for on-line measurement of contractions of single smooth muscle fibers under current or voltage clamp. Pflugers Arch. 1994;429:126–133. - PubMed
-
- Brezina V, Weiss KR. Analyzing the functional consequences of transmitter complexity. Trends Neurosci. 1997;20:538–543. - PubMed
-
- Brezina V, Evans CG, Weiss KR. Characterization of the membrane ion currents of a model molluscan muscle, the accessory radula closer muscle of Aplysia california. I. Hyperpolarization-activated currents. J Neurophysiol. 1994a;71:2093–2112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
