Mechanisms of spatial attention control in frontal and parietal cortex
- PMID: 20053897
- PMCID: PMC2809378
- DOI: 10.1523/JNEUROSCI.3862-09.2010
Mechanisms of spatial attention control in frontal and parietal cortex
Abstract
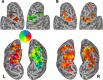
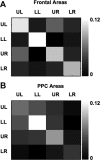
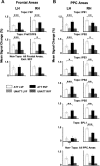
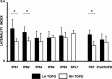
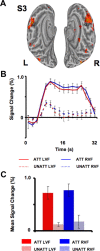
Theories of spatial attentional control have been largely based upon studies of patients suffering from visuospatial neglect, resulting from circumscribed lesions of frontal and posterior parietal cortex. In the intact brain, the control of spatial attention has been related to a distributed frontoparietal attention network. Little is known about the nature of the control mechanisms exerted by this network. Here, we used a novel region-of-interest approach to relate activations of the attention network to recently described topographic areas in frontal cortex [frontal eye field (FEF), PreCC/IFS (precentral cortex/inferior frontal sulcus)] and parietal cortex [intraparietal sulcus areas (IPS1-IPS5) and an area in the superior parietal lobule (SPL1)] to examine their spatial attention signals. We found that attention signals in most topographic areas were spatially specific, with stronger responses when attention was directed to the contralateral than to the ipsilateral visual field. Importantly, two hemispheric asymmetries were found. First, a region in only right, but not left SPL1 carried spatial attention signals. Second, left FEF and left posterior parietal cortex (IPS1/2) generated stronger contralateral biasing signals than their counterparts in the right hemisphere. These findings are the first to characterize spatial attention signals in topographic frontal and parietal cortex and provide a neural basis in support of an interhemispheric competition account of spatial attentional control.
Figures




Similar articles
-
Influences of Long-Term Memory-Guided Attention and Stimulus-Guided Attention on Visuospatial Representations within Human Intraparietal Sulcus.J Neurosci. 2015 Aug 12;35(32):11358-63. doi: 10.1523/JNEUROSCI.1055-15.2015. J Neurosci. 2015. PMID: 26269642 Free PMC article.
-
Dynamic activation of frontal, parietal, and sensory regions underlying anticipatory visual spatial attention.J Neurosci. 2011 Sep 28;31(39):13880-9. doi: 10.1523/JNEUROSCI.1519-10.2011. J Neurosci. 2011. PMID: 21957250 Free PMC article.
-
Shifting attentional priorities: control of spatial attention through hemispheric competition.J Neurosci. 2013 Mar 20;33(12):5411-21. doi: 10.1523/JNEUROSCI.4089-12.2013. J Neurosci. 2013. PMID: 23516306 Free PMC article.
-
Maps of space in human frontoparietal cortex.J Physiol Paris. 2013 Dec;107(6):510-6. doi: 10.1016/j.jphysparis.2013.04.002. Epub 2013 Apr 18. J Physiol Paris. 2013. PMID: 23603831 Free PMC article. Review.
-
The hybrid model of attentional control: New insights into hemispheric asymmetries inferred from TMS research.Neuropsychologia. 2015 Jul;74:21-9. doi: 10.1016/j.neuropsychologia.2014.11.023. Epub 2014 Nov 20. Neuropsychologia. 2015. PMID: 25451041 Review.
Cited by
-
The neural correlates of volitional attention: A combined fMRI and ERP study.Hum Brain Mapp. 2015 Jul;36(7):2443-54. doi: 10.1002/hbm.22783. Epub 2015 Mar 2. Hum Brain Mapp. 2015. PMID: 25731128 Free PMC article.
-
The impact of top-down spatial attention on laterality and hemispheric asymmetry in the human parietal cortex.J Vis. 2016 Aug 1;16(10):2. doi: 10.1167/16.10.2. J Vis. 2016. PMID: 27494544 Free PMC article.
-
Functions of the human frontoparietal attention network: Evidence from neuroimaging.Curr Opin Behav Sci. 2015 Feb;1:32-39. doi: 10.1016/j.cobeha.2014.08.003. Epub 2014 Aug 30. Curr Opin Behav Sci. 2015. PMID: 27398396 Free PMC article.
-
Mapping reflexive shifts of attention in eye-centered and hand-centered coordinate systems.Hum Brain Mapp. 2012 Jan;33(1):165-78. doi: 10.1002/hbm.21202. Epub 2011 Mar 9. Hum Brain Mapp. 2012. PMID: 21391262 Free PMC article.
-
Asymmetry of parietal interhemispheric connections in humans.J Neurosci. 2011 Jun 15;31(24):8967-75. doi: 10.1523/JNEUROSCI.6567-10.2011. J Neurosci. 2011. PMID: 21677180 Free PMC article.
References
-
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. - PubMed
-
- Bartolomeo P, Chokron S, Gainotti G. Laterally directed arm movements and right unilateral neglect after left hemisphere damage. Neuropsychologia. 2001;39:1013–1021. - PubMed
-
- Beis JM, Keller C, Morin N, Bartolomeo P, Bernati T, Chokron S, Leclercq M, Louis-Dreyfus A, Marchal F, Martin Y, Perennou D, Pradat-Diehl P, Prairial C, Rode G, Rousseaux M, Samuel C, Sieroff E, Wiart L, Azouvi P. Right spatial neglect after left hemisphere stroke: qualitative and quantitative study. Neurology. 2004;63:1600–1605. - PubMed
-
- Bon L, Lucchetti C. Attention-related neurons in the supplementary eye field of the macaque monkey. Exp Brain Res. 1997;113:180–185. - PubMed
-
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
