Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury
- PMID: 20053907
- PMCID: PMC2823089
- DOI: 10.1523/JNEUROSCI.3705-09.2010
Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury
Abstract
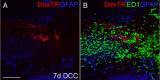
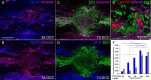
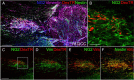
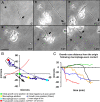
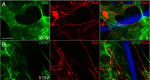
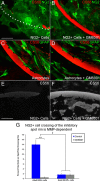
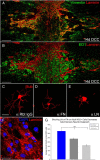
We previously demonstrated that activated ED1+ macrophages induce extensive axonal dieback of dystrophic sensory axons in vivo and in vitro. Interestingly, after spinal cord injury, the regenerating front of axons is typically found in areas rich in ED1+ cells, but devoid of reactive astrocyte processes. These observations suggested that another cell type must be present in these areas to counteract deleterious effects of macrophages. Cells expressing the purportedly inhibitory chondroitin sulfate proteoglycan NG2 proliferate in the lesion and intermingle with macrophages, but their influence on regeneration is highly controversial. Our in vivo analysis of dorsal column crush lesions confirms the close association between NG2+ cells and injured axons. We hypothesized that NG2+ cells were growth promoting and thereby served to increase axonal stability following spinal cord injury. We observed that the interactions between dystrophic adult sensory neurons and primary NG2+ cells derived from the adult spinal cord can indeed stabilize the dystrophic growth cone during macrophage attack. NG2+ cells expressed high levels of laminin and fibronectin, which promote neurite outgrowth on the surface of these cells. Our data also demonstrate that NG2+ cells, but not astrocytes, use matrix metalloproteases to extend across a region of inhibitory proteoglycan, and provide a permissive bridge for adult sensory axons. These data support the hypothesis that NG2+ cells are not inhibitory to regenerating sensory axons and, in fact, they may provide a favorable substrate that can stabilize the regenerating front of dystrophic axons in the inhibitory environment of the glial scar.
Figures


References
-
- Ben-Hur T, Ben-Yosef Y, Mizrachi-Kol R, Ben-Menachem O, Miller A. Cytokine-mediated modulation of MMPs and TIMPs in multipotential neural precursor cells. J Neuroimmunol. 2006;175:12–18. - PubMed
-
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. - PubMed
-
- Bodega G, Suárez I, Rubio M, Fernández B. Ependyma: phylogenetic evolution of glial fibrillary acidic protein (GFAP) and vimentin expression in vertebrate spinal cord. Histochemistry. 1994;102:113–122. - PubMed
-
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
