Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction
- PMID: 20053911
- PMCID: PMC2844797
- DOI: 10.1523/JNEUROSCI.4256-09.2010
Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction
Abstract
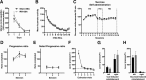
Drugs of abuse dynamically regulate adult neurogenesis, which appears important for some types of learning and memory. Interestingly, a major site of adult neurogenesis, the hippocampus, is important in the formation of drug-context associations and in the mediation of drug-taking and drug-seeking behaviors in animal models of addiction. Correlative evidence suggests an inverse relationship between hippocampal neurogenesis and drug-taking or drug-seeking behaviors, but the lack of a causative link has made the relationship between adult-generated neurons and addiction unclear. We used rat intravenous cocaine self-administration in rodents, a clinically relevant animal model of addiction, to test the hypothesis that suppression of adult hippocampal neurogenesis enhances vulnerability to addiction and relapse. Suppression of adult hippocampal neurogenesis via cranial irradiation before drug-taking significantly increased cocaine self-administration on both fixed-ratio and progressive-ratio schedules, as well as induced a vertical shift in the dose-response curve. This was not a general enhancement of learning, motivation, or locomotion, because sucrose self-administration and locomotor activity were unchanged in irradiated rats. Suppression of adult hippocampal neurogenesis after drug-taking significantly enhanced resistance to extinction of drug-seeking behavior. These studies identify reduced adult hippocampal neurogenesis as a novel risk factor for addiction-related behaviors in an animal model of cocaine addiction. Furthermore, they suggest that therapeutics to specifically increase or stabilize adult hippocampal neurogenesis could aid in preventing initial addiction as well as future relapse.
Figures






References
-
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. - PubMed
-
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. - PubMed
-
- Baker DA, Tran-Nguyen TL, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2001;155:18–26. - PubMed
-
- Borders AS, Getchell ML, Etscheidt JT, van Rooijen N, Cohen DA, Getchell TV. Macrophage depletion in the murine olfactory epithelium leads to increased neuronal death and decreased neurogenesis. J Comp Neurol. 2007;501:206–218. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
