Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington's disease
- PMID: 20053912
- PMCID: PMC6632544
- DOI: 10.1523/JNEUROSCI.4974-09.2010
Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington's disease
Abstract
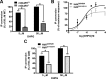
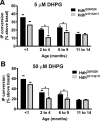
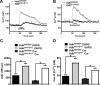
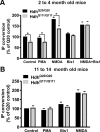
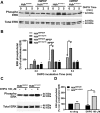
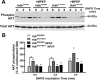
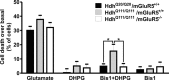
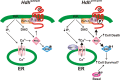
Huntington's disease (HD) is an autosomal-dominant neurodegenerative disorder caused by a polyglutamine expansion in the huntingtin protein (Htt). Group I metabotropic glutamate receptors (mGluRs) are coupled to G(alphaq) and play an important role in neuronal survival. We have previously demonstrated that mGluRs interact with Htt. Here we used striatal neuronal primary cultures and acute striatal slices to demonstrate that mGluR-mediated signaling pathways are altered in a presymptomatic mouse model of HD (Hdh(Q111/Q111)), as compared to those of control mice (Hdh(Q20/Q20)). mGluR1/5-mediated inositol phosphate (InsP) formation is desensitized in striatal slices from Hdh(Q111/Q111) mice and this desensitization is PKC-mediated. Despite of decreased InsP formation, (S)-3,5-dihydroxylphenylglycine (DHPG)-mediated Ca(2+) release is higher in Hdh(Q111/Q111) than in Hdh(Q20/Q20) neurons. Furthermore, mGluR1/5-stimulated AKT and extracellular signal-regulated kinase (ERK) activation is altered in Hdh(Q111/Q111) mice. Basal AKT activation is higher in Hdh(Q111/Q111) neurons and this increase is mGluR5 dependent. Moreover, mGluR5 activation leads to higher levels of ERK activation in Hdh(Q111/Q111) than in Hdh(Q20/Q20) striatum. PKC inhibition not only brings Hdh(Q111/Q111) DHPG-stimulated InsP formation to Hdh(Q20/Q20) levels, but also causes an increase in neuronal cell death in Hdh(Q111/Q111) neurons. However, PKC inhibition does not modify neuronal cell death in Hdh(Q20/Q20) neurons, suggesting that PKC-mediated desensitization of mGluR1/5 in Hdh(Q111/Q111) mice might be protective in HD. Together, these data indicate that group I mGluR-mediated signaling pathways are altered in HD and that these cell signaling adaptations could be important for striatal neurons survival.
Figures
References
-
- Alagarsamy S, Marino MJ, Rouse ST, Gereau RW, 4th, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci. 1999;2:234–240. - PubMed
-
- Anborgh PH, Godin C, Pampillo M, Dhami GK, Dale LB, Cregan SP, Truant R, Ferguson SS. Inhibition of metabotropic glutamate receptor signaling by the huntingtin-binding protein optineurin. J Biol Chem. 2005;280:34840–34848. - PubMed
-
- Baskys A, Bayazitov I, Fang L, Blaabjerg M, Poulsen FR, Zimmer J. Group I metabotropic glutamate receptors reduce excitotoxic injury and may facilitate neurogenesis. Neuropharmacology. 2005;49(Suppl 1):146–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
