Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex
- PMID: 20053914
- PMCID: PMC2823134
- DOI: 10.1523/JNEUROSCI.4554-09.2010
Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex
Abstract
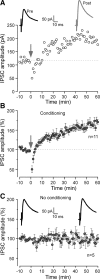
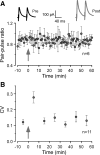
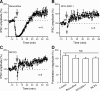
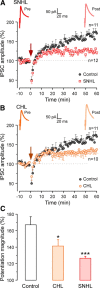
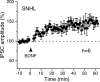
Long-term synaptic plasticity is a putative mechanism for learning in adults. However, there is little understanding of how synaptic plasticity mechanisms develop or whether their maturation depends on experience. Since inhibitory synapses are particularly malleable to sensory stimulation, long-lasting potentiation of inhibitory synapses was characterized in auditory thalamocortical slices. Intracortical high-frequency electrical stimulation led to a 67% increase in inhibitory synaptic currents. In the absence of stimulation, inhibitory potentiation was induced by a brief exposure to exogenous brain-derived neurotrophic factor (BDNF). BDNF exposure occluded any additional potentiation by high-frequency afferent stimulation, suggesting that BDNF signaling is sufficient to account for inhibitory potentiation. Moreover, inhibitory potentiation was reduced significantly by extracellular application of a BDNF scavenger or by intracellular blockade of BDNF receptor [tropomyosin-related kinase B (TrkB)] signaling. In contrast, glutamatergic or GABAergic antagonists did not prevent the induction of inhibitory potentiation. Since BDNF and TrkB expression are influenced strongly by activity, we predicted that inhibitory potentiation would be diminished by manipulations that decrease central auditory activity, such as hearing loss. Two forms of hearing loss were examined: conductive hearing loss in which the cochleae are not damaged or sensorineural hearing loss in which both cochleae are removed. Both forms of hearing loss were found to reduce significantly the magnitude of inhibitory potentiation. These data indicate that early experience is necessary for the normal development of BDNF-mediated long-lasting inhibitory potentiation, which may be associated with perceptual deficits at later ages.
Figures



Similar articles
-
Age-dependent effect of hearing loss on cortical inhibitory synapse function.J Neurophysiol. 2012 Feb;107(3):937-47. doi: 10.1152/jn.00515.2011. Epub 2011 Nov 16. J Neurophysiol. 2012. PMID: 22090457 Free PMC article.
-
Continuous white noise exposure during and after auditory critical period differentially alters bidirectional thalamocortical plasticity in rat auditory cortex in vivo.Eur J Neurosci. 2007 Nov;26(9):2576-84. doi: 10.1111/j.1460-9568.2007.05857.x. Epub 2007 Oct 26. Eur J Neurosci. 2007. PMID: 17970743
-
Preserving Inhibition during Developmental Hearing Loss Rescues Auditory Learning and Perception.J Neurosci. 2019 Oct 16;39(42):8347-8361. doi: 10.1523/JNEUROSCI.0749-19.2019. Epub 2019 Aug 26. J Neurosci. 2019. PMID: 31451577 Free PMC article.
-
BDNF mechanisms in late LTP formation: A synthesis and breakdown.Neuropharmacology. 2014 Jan;76 Pt C:664-76. doi: 10.1016/j.neuropharm.2013.06.024. Epub 2013 Jul 2. Neuropharmacology. 2014. PMID: 23831365 Review.
-
Developmental plasticity of auditory cortical inhibitory synapses.Hear Res. 2011 Sep;279(1-2):140-8. doi: 10.1016/j.heares.2011.03.015. Epub 2011 Apr 2. Hear Res. 2011. PMID: 21463668 Free PMC article. Review.
Cited by
-
Auditory training during development mitigates a hearing loss-induced perceptual deficit.Front Syst Neurosci. 2014 Apr 4;8:49. doi: 10.3389/fnsys.2014.00049. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24772071 Free PMC article.
-
Advances in the Electrophysiological Recordings of Long-Term Potentiation.Int J Mol Sci. 2023 Apr 12;24(8):7134. doi: 10.3390/ijms24087134. Int J Mol Sci. 2023. PMID: 37108295 Free PMC article. Review.
-
Deletion of BDNF in Pax2 Lineage-Derived Interneuron Precursors in the Hindbrain Hampers the Proportion of Excitation/Inhibition, Learning, and Behavior.Front Mol Neurosci. 2021 Mar 26;14:642679. doi: 10.3389/fnmol.2021.642679. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33841098 Free PMC article.
-
A sensitive period for the impact of hearing loss on auditory perception.J Neurosci. 2014 Feb 5;34(6):2276-84. doi: 10.1523/JNEUROSCI.0647-13.2014. J Neurosci. 2014. PMID: 24501366 Free PMC article.
-
Age-dependent effect of hearing loss on cortical inhibitory synapse function.J Neurophysiol. 2012 Feb;107(3):937-47. doi: 10.1152/jn.00515.2011. Epub 2011 Nov 16. J Neurophysiol. 2012. PMID: 22090457 Free PMC article.
References
-
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Androutsellis-Theotokis A, McCormack WJ, Bradford HF, Stern GM, Pliego-Rivero FB. The depolarisation-induced release of [125I]BDNF from brain tissue. Brain Res. 1996;743:40–48. - PubMed
-
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol. 2007;17:325–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
