Functional, but not anatomical, separation of "what" and "when" in prefrontal cortex
- PMID: 20053916
- PMCID: PMC2947945
- DOI: 10.1523/JNEUROSCI.3276-09.2010
Functional, but not anatomical, separation of "what" and "when" in prefrontal cortex
Abstract
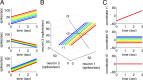
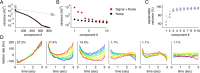
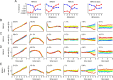
How does the brain store information over a short period of time? Typically, the short-term memory of items or values is thought to be stored in the persistent activity of neurons in higher cortical areas. However, the activity of these neurons often varies strongly in time, even if time is unimportant for whether or not rewards are received. To elucidate this interaction of time and memory, we reexamined the activity of neurons in the prefrontal cortex of monkeys performing a working memory task. As often observed in higher cortical areas, different neurons have highly heterogeneous patterns of activity, making interpretation of the data difficult. To overcome these problems, we developed a method that finds a new representation of the data in which heterogeneity is much reduced, and time- and memory-related activities became separate and easily interpretable. This new representation consists of a few fundamental activity components that capture 95% of the firing rate variance of >800 neurons. Surprisingly, the memory-related activity components account for <20% of this firing rate variance. The observed heterogeneity of neural responses results from random combinations of these fundamental components. Based on these components, we constructed a generative linear model of the network activity. The model suggests that the representations of time and memory are maintained by separate mechanisms, even while sharing a common anatomical substrate. Testable predictions of this hypothesis are proposed. We suggest that our method may be applied to data from other tasks in which neural responses are highly heterogeneous across neurons, and dependent on more than one variable.
Figures





Similar articles
-
Encoding of Serial Order in Working Memory: Neuronal Activity in Motor, Premotor, and Prefrontal Cortex during a Memory Scanning Task.J Neurosci. 2018 May 23;38(21):4912-4933. doi: 10.1523/JNEUROSCI.3294-17.2018. Epub 2018 Apr 30. J Neurosci. 2018. PMID: 29712786 Free PMC article.
-
Monkey prefrontal neurons during Sternberg task performance: full contents of working memory or most recent item?J Neurophysiol. 2017 Jun 1;117(6):2269-2281. doi: 10.1152/jn.00541.2016. Epub 2017 Mar 22. J Neurophysiol. 2017. PMID: 28331006 Free PMC article.
-
Persistent discharges in the prefrontal cortex of monkeys naive to working memory tasks.Cereb Cortex. 2007 Sep;17 Suppl 1:i70-6. doi: 10.1093/cercor/bhm063. Cereb Cortex. 2007. PMID: 17726005
-
Prefrontal cortex and working memory processes.Neuroscience. 2006 Apr 28;139(1):251-61. doi: 10.1016/j.neuroscience.2005.07.003. Epub 2005 Dec 1. Neuroscience. 2006. PMID: 16325345 Review.
-
Prefrontal cortex and neural mechanisms of executive function.J Physiol Paris. 2013 Dec;107(6):471-82. doi: 10.1016/j.jphysparis.2013.05.001. Epub 2013 May 15. J Physiol Paris. 2013. PMID: 23684970 Review.
Cited by
-
Comparison of neural activity related to working memory in primate dorsolateral prefrontal and posterior parietal cortex.Front Syst Neurosci. 2010 May 14;4:12. doi: 10.3389/fnsys.2010.00012. eCollection 2010. Front Syst Neurosci. 2010. PMID: 20514341 Free PMC article.
-
A Neural Population Mechanism for Rapid Learning.Neuron. 2018 Nov 21;100(4):964-976.e7. doi: 10.1016/j.neuron.2018.09.030. Epub 2018 Oct 18. Neuron. 2018. PMID: 30344047 Free PMC article.
-
Estimating the dimensionality of the manifold underlying multi-electrode neural recordings.PLoS Comput Biol. 2021 Nov 29;17(11):e1008591. doi: 10.1371/journal.pcbi.1008591. eCollection 2021 Nov. PLoS Comput Biol. 2021. PMID: 34843461 Free PMC article.
-
Timing over tuning: overcoming the shortcomings of a line attractor during a working memory task.PLoS Comput Biol. 2014 Jan 30;10(1):e1003437. doi: 10.1371/journal.pcbi.1003437. eCollection 2014 Jan. PLoS Comput Biol. 2014. PMID: 24499929 Free PMC article.
-
Optogenetic perturbations reveal the dynamics of an oculomotor integrator.Front Neural Circuits. 2014 Feb 28;8:10. doi: 10.3389/fncir.2014.00010. eCollection 2014. Front Neural Circuits. 2014. PMID: 24616666 Free PMC article.
References
-
- Brody CD, Hernández A, Zainos A, Romo R. Timing and neural encoding of somatosensory parametric working memory in macaque prefrontal cortex. Cereb Cortex. 2003;13:1196–1207. - PubMed
-
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. - PubMed
-
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
