Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy
- PMID: 20053921
- PMCID: PMC2903060
- DOI: 10.1523/JNEUROSCI.4093-09.2010
Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy
Abstract
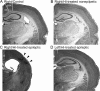
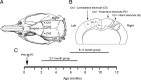
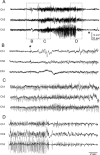
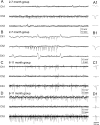
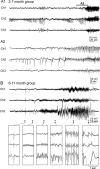
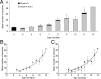
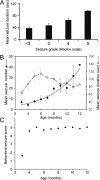
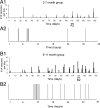
The development of acquired epilepsy after a perinatal hypoxic-ischemic (HI) insult was investigated in rats. After unilateral carotid ligation with hypoxia on postnatal day 7, cortical electroencephalographic and behavioral seizures were recorded with continuous radio-telemetry and video. Chronic recordings were obtained between 2 and 12 months of age in freely behaving HI-treated and sham control rats. The hypotheses were that the acquired epilepsy is directly associated with an ischemic infarct (i.e., no lesion, no epilepsy), and the resultant epilepsy is temporally progressive. Every HI-treated rat with a cerebral infarct developed spontaneous epileptiform discharges and recurrent seizures (100%); in contrast, no spontaneous epileptiform discharges or seizures were detected with continuous monitoring in the HI-treated rats without infarcts. The initial seizures at 2 months generally showed focal onset and were nonconvulsive. Subsequent seizures had focal onsets that propagated to the homotopic contralateral cortex and were nonconvulsive or partial; later seizures often appeared to have bilateral onset and were convulsive. Spontaneous epileptiform discharges were initially lateralized to ipsilateral neocortex but became bilateral over time. The severity and frequency of the spontaneous behavioral and electrographic seizures progressively increased over time. In every epileptic rat, seizures occurred in distinct clusters with seizure-free periods as long as a few weeks. The progressive increase in seizure frequency over time was associated with increases in cluster frequency and seizures within each cluster. Thus, prolonged, continuous seizure monitoring directly demonstrated that the acquired epilepsy after perinatal HI was progressive with seizure clusters and was consistently associated with a cerebral infarct.
Figures
Similar articles
-
Neuropathogical features of a rat model for perinatal hypoxic-ischemic encephalopathy with associated epilepsy.J Comp Neurol. 2007 Dec 20;505(6):716-37. doi: 10.1002/cne.21533. J Comp Neurol. 2007. PMID: 17948865 Free PMC article.
-
Ischemic injury suppresses hypoxia-induced electrographic seizures and the background EEG in a rat model of perinatal hypoxic-ischemic encephalopathy.J Neurophysiol. 2015 Nov;114(5):2753-63. doi: 10.1152/jn.00796.2014. Epub 2015 Sep 9. J Neurophysiol. 2015. PMID: 26354320 Free PMC article.
-
Background suppression of electrical activity is a potential biomarker of subsequent brain injury in a rat model of neonatal hypoxia-ischemia.J Neurophysiol. 2022 Jul 1;128(1):118-130. doi: 10.1152/jn.00024.2022. Epub 2022 Jun 8. J Neurophysiol. 2022. PMID: 35675445 Free PMC article.
-
The time course of acquired epilepsy: implications for therapeutic intervention to suppress epileptogenesis.Neurosci Lett. 2011 Jun 27;497(3):240-6. doi: 10.1016/j.neulet.2011.03.071. Epub 2011 Mar 31. Neurosci Lett. 2011. PMID: 21458536 Review.
-
Development of spontaneous seizures after experimental status epilepticus: implications for understanding epileptogenesis.Epilepsia. 2007;48 Suppl 5:157-63. doi: 10.1111/j.1528-1167.2007.01304.x. Epilepsia. 2007. PMID: 17910596 Review.
Cited by
-
Interictal Discharges: All Roads Lead to Rome?Epilepsy Curr. 2022 Jul 16;22(4):252-254. doi: 10.1177/15357597221098809. eCollection 2022 Jul-Aug. Epilepsy Curr. 2022. PMID: 36187148 Free PMC article. No abstract available.
-
A mouse model of seizures in anti-N-methyl-d-aspartate receptor encephalitis.Epilepsia. 2019 Mar;60(3):452-463. doi: 10.1111/epi.14662. Epub 2019 Feb 11. Epilepsia. 2019. PMID: 30740690 Free PMC article.
-
Does acquired epileptogenesis in the immature brain require neuronal death.Epilepsy Curr. 2011 Jan;11(1):21-6. doi: 10.5698/1535-7511-11.1.21. Epilepsy Curr. 2011. PMID: 21461261 Free PMC article.
-
Seizure activity occurs in the collagenase but not the blood infusion model of striatal hemorrhagic stroke in rats.Transl Stroke Res. 2015 Feb;6(1):29-38. doi: 10.1007/s12975-014-0361-y. Epub 2014 Jul 24. Transl Stroke Res. 2015. PMID: 25053257 Free PMC article.
-
WONOEP appraisal: new genetic approaches to study epilepsy.Epilepsia. 2014 Aug;55(8):1170-86. doi: 10.1111/epi.12692. Epub 2014 Jun 25. Epilepsia. 2014. PMID: 24965021 Free PMC article. Review.
References
-
- Aneja S, Ahuja B, Taluja V, Bhatia VK. Epilepsy in children with cerebral palsy. Indian J Pediatr. 2001;68:111–115. - PubMed
-
- Badawi N, Felix JF, Kurinczuk JJ, Dixon G, Watson L, Keogh JM, Valentine J, Stanley FJ. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47:293–298. - PubMed
-
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. - PubMed
-
- Bergamasco B, Benna P, Ferrero P, Gavinelli R. Neonatal hypoxia and epileptic risk: a clinical prospective study. Epilepsia. 1984;25:131–136. - PubMed
-
- Bertram EH, Cornett J. The ontogeny of seizures in a rat model of limbic epilepsy: evidence for a kindling process in the development of chronic spontaneous seizures. Brain Res. 1993;625:295–300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
