Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening
- PMID: 20053924
- PMCID: PMC2838564
- DOI: 10.1152/ajpcell.00468.2009
Mitochondrial matrix K+ flux independent of large-conductance Ca2+-activated K+ channel opening
Abstract
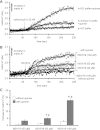
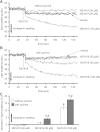
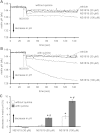
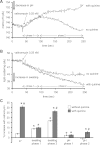
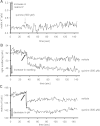
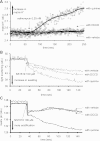
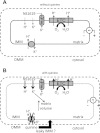
Large-conductance Ca(2+)-activated K(+) channels (BK(Ca)) in the inner mitochondrial membrane may play a role in protecting against cardiac ischemia-reperfusion injury. NS1619 (30 microM), an activator of BK(Ca) channels, was shown to increase respiration and to stimulate reactive oxygen species generation in isolated cardiac mitochondria energized with succinate. Here, we tested effects of NS1619 to alter matrix K(+), H(+), and swelling in mitochondria isolated from guinea pig hearts. We found that 30 microM NS1619 did not change matrix K(+), H(+), and swelling, but that 50 and 100 microM NS1619 caused a concentration-dependent increase in matrix K(+) influx (PBFI fluorescence) only when quinine was present to block K(+)/H(+) exchange (KHE); this was accompanied by increased mitochondrial matrix volume (light scattering). Matrix pH (BCECF fluorescence) was decreased slightly by 50 and 100 microM NS1619 but markedly more so when quinine was present. NS1619 (100 microM) caused a significant leak in lipid bilayers, and this was enhanced in the presence of quinine. The K(+) ionophore valinomycin (0.25 nM), which like NS1619 increased matrix volume and increased K(+) influx in the presence of quinine, caused matrix alkalinization followed by acidification when quinine was absent, and only alkalinization when quinine was present. If K(+) is exchanged instantly by H(+) through activated KHE, then matrix K(+) influx should stimulate H(+) influx through KHE and cause matrix acidification. Our results indicate that KHE is not activated immediately by NS1619-induced K(+) influx, that NS1619 induces matrix K(+) and H(+) influx through a nonspecific transport mechanism, and that enhancement with quinine is not due to the blocking of KHE, but to a nonspecific effect of quinine to enhance current leak by NS1619.
Figures

References
-
- Armando-Hardy M, Ellory JC, Ferreira HG, Fleminger S, Lew VL. Inhibition of the calcium-induced increase in the potassium permeability of human red blood cells by quinine. J Physiol 250: 32P–33P, 1975 - PubMed
-
- Azzi A, Casey RP, Nalecz MJ. The effect of N,N′-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta 768: 209–226, 1984 - PubMed
-
- Azzi A, Scarpa A. Inhibition of K+ transport in liver mitochondria. Biochim Biophys Acta 135: 1087–1088, 1967 - PubMed
-
- Azzone GF, Bortolotto F, Zanotti A. Induction of electroneutral exchanges of H+ with K+ in rat liver mitochondria. FEBS Lett 96: 135–140, 1978 - PubMed
-
- Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix volume. J Biol Chem 260: 13424–13433, 1985 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

