Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells
- PMID: 20053980
- PMCID: PMC2891460
- DOI: 10.1167/iovs.09-4874
Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells
Abstract
Purpose: To investigate the alterations in microRNA (miRNA) expression during replicative senescence (RS) in human trabecular meshwork (HTM) cells.
Methods: Two HTM cell lines were serially passaged until they reached RS. Changes in expression of 30 miRNAs were assessed by real-time quantitative (q)-PCR. The effects of miR-146a on gene expression were analyzed with gene arrays and the results confirmed by real-time q-PCR. Protein levels of IRAK1 and PAI-1 were analyzed by Western blot and those of IL6 and IL8 by ELISA. Senescence-associated markers were monitored by flow cytometry and cell proliferation by BrdU incorporation.
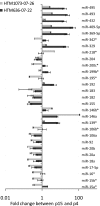
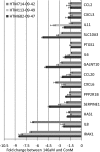
Results: RS of HTM cells was associated with significant changes in expression of 18 miRNAs, including the upregulation of miR-146a. miR-146a downregulated multiple genes associated with inflammation, including IRAK1, IL6, IL8, and PAI-1, inhibited senescence-associated beta-galactosidase (SA-beta-gal) activity and production of intracellular reactive species (iROS), and increased cell proliferation. Overexpression of either IRAK1 or PAI-1 inhibited the effects of miR-146a on cell proliferation and iROS production in senescent cells.
Conclusions: RS in HTM cells was associated with changes in miRNA expression that could influence the senescent phenotype. Upregulation of the anti-inflammatory miR-146a may serve to restrain excessive production of inflammatory mediators in senescent cells and limit their deleterious effects on the surrounding tissue. Among the different proteins repressed by miR-146a, the inhibition of PAI-1 may act to minimize the effects of senescence on the generation of iROS and growth arrest and prevent alterations of the extracellular proteolytic activity of the TM.
Figures





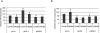
Similar articles
-
Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells.Invest Ophthalmol Vis Sci. 2011 May 6;52(6):2999-3007. doi: 10.1167/iovs.10-6708. Invest Ophthalmol Vis Sci. 2011. PMID: 21282569 Free PMC article.
-
MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8.Aging (Albany NY). 2009 Apr;1(4):402-11. doi: 10.18632/aging.100042. Aging (Albany NY). 2009. PMID: 20148189 Free PMC article.
-
Alterations in microRNA expression in stress-induced cellular senescence.Mech Ageing Dev. 2009 Nov-Dec;130(11-12):731-41. doi: 10.1016/j.mad.2009.09.002. Mech Ageing Dev. 2009. PMID: 19782699 Free PMC article.
-
MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling.Age (Dordr). 2013 Aug;35(4):1157-72. doi: 10.1007/s11357-012-9440-8. Epub 2012 Jun 13. Age (Dordr). 2013. PMID: 22692818 Free PMC article.
-
Senescence-Associated miRNAs and Their Role in Pancreatic Cancer.Pathol Oncol Res. 2022 Apr 29;28:1610156. doi: 10.3389/pore.2022.1610156. eCollection 2022. Pathol Oncol Res. 2022. PMID: 35570840 Free PMC article. Review.
Cited by
-
Aging, Cellular Senescence, and Glaucoma.Aging Dis. 2024 Apr 1;15(2):546-564. doi: 10.14336/AD.2023.0630-1. Aging Dis. 2024. PMID: 37725658 Free PMC article. Review.
-
The role of microRNAs in glaucoma.Exp Eye Res. 2022 Feb;215:108909. doi: 10.1016/j.exer.2021.108909. Epub 2021 Dec 27. Exp Eye Res. 2022. PMID: 34968473 Free PMC article. Review.
-
miR-146a is upregulated during retinal pigment epithelium (RPE)/choroid aging in mice and represses IL-6 and VEGF-A expression in RPE cells.J Clin Exp Ophthalmol. 2016 Jun;7(3):562. doi: 10.4172/2155-9570.1000562. Epub 2016 Jun 24. J Clin Exp Ophthalmol. 2016. PMID: 27917303 Free PMC article.
-
miRNA Studies in Glaucoma: A Comprehensive Review of Current Knowledge and Future Perspectives.Int J Mol Sci. 2023 Sep 28;24(19):14699. doi: 10.3390/ijms241914699. Int J Mol Sci. 2023. PMID: 37834147 Free PMC article. Review.
-
Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells.Invest Ophthalmol Vis Sci. 2011 May 6;52(6):2999-3007. doi: 10.1167/iovs.10-6708. Invest Ophthalmol Vis Sci. 2011. PMID: 21282569 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

