Impact of tumor-associated macrophages in LH(BETA)T(AG) mice on retinal tumor progression: relation to macrophage subtype
- PMID: 20053982
- PMCID: PMC2868494
- DOI: 10.1167/iovs.09-4255
Impact of tumor-associated macrophages in LH(BETA)T(AG) mice on retinal tumor progression: relation to macrophage subtype
Abstract
Purpose: To determine the distribution of tumor-associated macrophages (TAMs) during retinoblastoma tumor development, examine the contribution of bone marrow-derived TAMs in retinoblastoma tumors, and evaluate the supportive role of TAMs in tumor growth in a transgenic retinoblastoma mouse model.
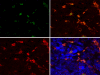
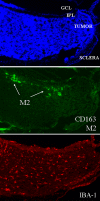
Methods: The time course of macrophage infiltration in transgenic retinoblastoma tumors was assessed by immunohistochemistry at different time points in tumorigenesis. The origin of TAMs in transgenic retinoblastoma tumors was determined by transplanting 10(7) bone marrow cells from green fluorescent protein (GFP)-positive 16-week-old mice into age-matched, irradiated LH(BETA)T(AG) mice via tail vein injections. Macrophage depletion was performed by subconjunctival (SC) delivery of liposomal clodronate.
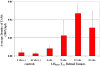
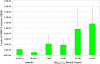
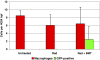
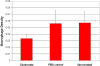
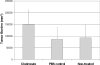
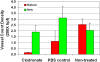
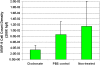
Results: The density of TAMs increased from 4 to 12 weeks of age in mice with small to medium tumors (P = 0.037) and remained stable in the later stages of disease (i.e., 16 weeks old with large tumors; P = 0.20). In 16-week-old mice, 38% (2.5 +/- 3.2 cells per 400x high-power field) of TAMs were GFP-positive, bone marrow-derived macrophages. Total TAM depletion was associated with a significant decrease in the expression levels of MMP-9 (P = 0.014) and mature vessels (P < 0.001) and a nonsignificant decrease in the density of neovessels (P = 0.94). The density of M2-polarized TAMs did not change significantly after TAM depletion (P = 0.68). After M1-polarized TAM depletion, the tumor burden increased (P = 0.056).
Conclusions: This work extends understanding of the complex role that macrophages play in retinoblastoma. Macrophage modulation in the tumor microenvironment is a critical factor in retinoblastoma tumor progression.
Figures
Similar articles
-
Blood vessel maturation in retinoblastoma tumors: spatial distribution of neovessels and mature vessels and its impact on ocular treatment.Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1020-4. doi: 10.1167/iovs.08-2654. Epub 2008 Oct 24. Invest Ophthalmol Vis Sci. 2009. PMID: 18952925
-
Retinoblastoma tumor vessel maturation impacts efficacy of vessel targeting in the LH(BETA)T(AG) mouse model.Invest Ophthalmol Vis Sci. 2007 Jun;48(6):2476-82. doi: 10.1167/iovs.06-1397. Invest Ophthalmol Vis Sci. 2007. PMID: 17525173
-
Gelatinase expression in retinoblastoma: modulation of LH(BETA)T(AG) retinal tumor development by anecortave acetate.Invest Ophthalmol Vis Sci. 2010 Jun;51(6):2860-4. doi: 10.1167/iovs.09-4500. Epub 2010 Jan 27. Invest Ophthalmol Vis Sci. 2010. PMID: 20107171 Free PMC article.
-
Retinoblastoma in transgenic mice: models of hereditary retinoblastoma.Surv Ophthalmol. 1999 May-Jun;43(6):508-18. doi: 10.1016/s0039-6257(99)00047-8. Surv Ophthalmol. 1999. PMID: 10416793 Review.
-
The role of tumor-associated macrophage in breast cancer biology.Histol Histopathol. 2018 Feb;33(2):133-145. doi: 10.14670/HH-11-916. Epub 2017 Jul 6. Histol Histopathol. 2018. PMID: 28681373 Review.
Cited by
-
Anti-Inflammatory Activation of Phellodendri Chinensis Cortex is Mediated by Berberine Erythrocytes Self-Assembly Targeted Delivery System.Drug Des Devel Ther. 2022 Dec 23;16:4365-4383. doi: 10.2147/DDDT.S385301. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36583113 Free PMC article.
-
Tumor Environment of Retinoblastoma, Intraocular Cancer.Adv Exp Med Biol. 2020;1296:349-358. doi: 10.1007/978-3-030-59038-3_21. Adv Exp Med Biol. 2020. PMID: 34185303
-
Optimizing CARs for ocular delivery.Nat Cancer. 2020 Oct;1(10):939-940. doi: 10.1038/s43018-020-00127-y. Epub 2020 Oct 12. Nat Cancer. 2020. PMID: 34693296 Free PMC article.
-
Evidence of Tumour Microenvironment and Stromal Cellular Components in Retinoblastoma.Ocul Oncol Pathol. 2019 Feb;5(2):85-93. doi: 10.1159/000488709. Epub 2018 Jul 17. Ocul Oncol Pathol. 2019. PMID: 30976585 Free PMC article.
-
The TAg-RB murine retinoblastoma cell of origin has immunohistochemical features of differentiated Muller glia with progenitor properties.Invest Ophthalmol Vis Sci. 2011 Sep 29;52(10):7618-24. doi: 10.1167/iovs.11-7989. Invest Ophthalmol Vis Sci. 2011. PMID: 21862643 Free PMC article.
References
-
- Pendergrass TW, Davis S. Incidence of retinoblastoma in the United States. Arch Ophthalmol 1980;98:1204–1210 - PubMed
-
- Tamboli A, Podgor MJ, Horm JW. The incidence of retinoblastoma in the United States: 1974 through 1985. Arch Ophthalmol 1990;108:128–132 - PubMed
-
- Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol 2004;122:1316–1323 - PubMed
-
- Khelfaoui F, Validire P, Auperin A, et al. Histopathologic risk factors in retinoblastoma: a retrospective study of 172 patients treated in a single institution. Cancer 1996;77:1206–1213 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

