Heterodimerization with different Jun proteins controls c-Fos intranuclear dynamics and distribution
- PMID: 20053986
- PMCID: PMC2825451
- DOI: 10.1074/jbc.M109.032680
Heterodimerization with different Jun proteins controls c-Fos intranuclear dynamics and distribution
Abstract
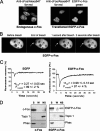
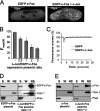
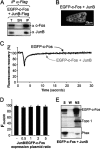
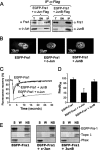
The c-Fos proto-oncogenic transcription factor defines a multigene family controlling many processes both at the cell and the whole organism level. To bind to its target AP-1/12-O-tetradecanoylphorbol-13-acetate-responsive element or cAMP-responsive element DNA sequences in gene promoters and exert its transcriptional part, c-Fos must heterodimerize with other bZip proteins, its best studied partners being the Jun proteins (c-Jun, JunB, and JunD). c-Fos expression is regulated at many transcriptional and post-transcriptional levels, yet little is known on how its localization is dynamically regulated in the cell. Here we have investigated its intranuclear mobility using fluorescence recovery after photobleaching, genetic, and biochemical approaches. Whereas monomeric c-Fos is highly mobile and distributed evenly with nucleolar exclusion in the nucleus, heterodimerization with c-Jun entails intranuclear redistribution and dramatic reduction in mobility of c-Fos caused by predominant association with the nuclear matrix independently of any binding to AP-1/12-O-tetradecanoylphorbol-13-acetate-responsive element or cAMP-responsive element sequences. In contrast to c-Jun, dimerization with JunB does not detectably affect c-Fos mobility. However, dimerization with JunB affects intranuclear distribution with significant differences in the localization of c-Fos.c-Jun and c-Fos.JunB dimers. Moreover, c-Jun and JunB exert comparable effects on another Fos family member, Fra-1. Thus, we report a novel regulation, i.e. differentially regulated intranuclear mobility and distribution of Fos proteins by their Jun partners, and suggest the existence of intranuclear storage sites for latent c-Fos.c-Jun AP-1 complexes. This may affect the numerous physiopathological functions these transcription factors control.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

