A tyrosine-based motif localizes a Drosophila vesicular transporter to synaptic vesicles in vivo
- PMID: 20053989
- PMCID: PMC2844137
- DOI: 10.1074/jbc.M109.073064
A tyrosine-based motif localizes a Drosophila vesicular transporter to synaptic vesicles in vivo
Abstract
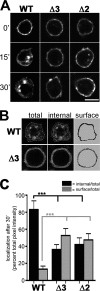
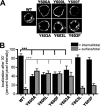
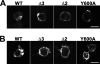
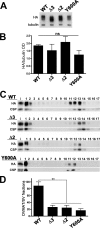
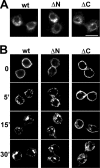
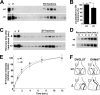
Vesicular neurotransmitter transporters must localize to synaptic vesicles (SVs) to allow regulated neurotransmitter release at the synapse. However, the signals required to localize vesicular proteins to SVs in vivo remain unclear. To address this question we have tested the effects of mutating proposed trafficking domains in Drosophila orthologs of the vesicular monoamine and glutamate transporters, DVMAT-A and DVGLUT. We show that a tyrosine-based motif (YXXY) is important both for DVMAT-A internalization from the cell surface in vitro, and localization to SVs in vivo. In contrast, DVGLUT deletion mutants that lack a putative C-terminal trafficking domain show more modest defects in both internalization in vitro and trafficking to SVs in vivo. Our data show for the first time that mutation of a specific trafficking motif can disrupt localization to SVs in vivo and suggest possible differences in the sorting of VMATs versus VGLUTs to SVs at the synapse.
Figures


Similar articles
-
The redistribution of Drosophila vesicular monoamine transporter mutants from synaptic vesicles to large dense-core vesicles impairs amine-dependent behaviors.J Neurosci. 2014 May 14;34(20):6924-37. doi: 10.1523/JNEUROSCI.0694-14.2014. J Neurosci. 2014. PMID: 24828646 Free PMC article.
-
Vesicular neurotransmitter transporter trafficking in vivo: moving from cells to flies.Fly (Austin). 2010 Oct-Dec;4(4):302-5. doi: 10.4161/fly.4.4.13305. Epub 2010 Oct 1. Fly (Austin). 2010. PMID: 20855951
-
A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine.J Neurobiol. 2005 Sep 5;64(3):239-58. doi: 10.1002/neu.20146. J Neurobiol. 2005. PMID: 15849736
-
Trafficking of vesicular neurotransmitter transporters.Traffic. 2008 Sep;9(9):1425-36. doi: 10.1111/j.1600-0854.2008.00771.x. Epub 2008 May 26. Traffic. 2008. PMID: 18507811 Free PMC article. Review.
-
Synaptic vesicle protein trafficking at the glutamate synapse.Neuroscience. 2009 Jan 12;158(1):189-203. doi: 10.1016/j.neuroscience.2008.03.029. Epub 2008 Mar 22. Neuroscience. 2009. PMID: 18472224 Free PMC article. Review.
Cited by
-
Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration.Neurobiol Dis. 2011 Feb;41(2):415-20. doi: 10.1016/j.nbd.2010.10.009. Epub 2010 Oct 14. Neurobiol Dis. 2011. PMID: 20951206 Free PMC article.
-
The redistribution of Drosophila vesicular monoamine transporter mutants from synaptic vesicles to large dense-core vesicles impairs amine-dependent behaviors.J Neurosci. 2014 May 14;34(20):6924-37. doi: 10.1523/JNEUROSCI.0694-14.2014. J Neurosci. 2014. PMID: 24828646 Free PMC article.
-
The Raf/LIN-45 C-terminal distal tail segment negatively regulates signaling in Caenorhabditis elegans.Genetics. 2024 Nov 6;228(3):iyae152. doi: 10.1093/genetics/iyae152. Genetics. 2024. PMID: 39288021
-
Restraint of presynaptic protein levels by Wnd/DLK signaling mediates synaptic defects associated with the kinesin-3 motor Unc-104.Elife. 2017 Sep 19;6:e24271. doi: 10.7554/eLife.24271. Elife. 2017. PMID: 28925357 Free PMC article.
-
Wnt signalosome assembly is governed by conformational flexibility of Axin and by the AP2 clathrin adaptor.Nat Commun. 2025 May 21;16(1):4718. doi: 10.1038/s41467-025-59984-9. Nat Commun. 2025. PMID: 40399324 Free PMC article.
References
-
- Hannah M. J., Schmidt A. A., Huttner W. B. (1999) Annu. Rev. Cell Dev. Biol. 15, 733–798 - PubMed
-
- Jahn R., Rizzoli S. O. (2007) Traffic 8, 1121–1122 - PubMed
-
- Barbosa J., Jr., Ferreira L. T., Martins-Silva C., Santos M. S., Torres G. E., Caron M. G., Gomez M. V., Ferguson S. S., Prado M. A., Prado V. F. (2002) J. Neurochem. 82, 1221–1228 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

