Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion
- PMID: 20053999
- PMCID: PMC2844179
- DOI: 10.1074/jbc.M109.091546
Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion
Abstract
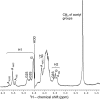
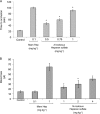
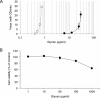
Heparin-like glycans with diverse disaccharide composition and high anticoagulant activity have been described in several families of marine mollusks. The present work focused on the structural characterization of a new heparan sulfate (HS)-like polymer isolated from the mollusk Nodipecten nodosus (Linnaeus, 1758) and on its anticoagulant and antithrombotic properties. Total glycans were extracted from the mollusk and fractionated by ethanol precipitation. The main component (>90%) was identified as HS-like glycosaminoglycan, representing approximately 4.6 mg g(-1) of dry tissue. The mollusk HS resists degradation with heparinase I but is cleaved by nitrous acid. Analysis of the mollusk glycan by one-dimensional (1)H, two-dimensional correlated spectroscopy, and heteronuclear single quantum coherence nuclear magnetic resonance revealed characteristic signals of glucuronic acid and glucosamine residues. Signals corresponding to anomeric protons of nonsulfated, 3- or 2-sulfated glucuronic acid as well as N-sulfated and/or 6-sulfated glucosamine were also observed. The mollusk HS has an anticoagulant activity of 36 IU mg(-1), 5-fold lower than porcine heparin (180 IU mg(-1)), as measured by the activated partial thromboplastin time assay. It also inhibits factor Xa (IC(50) = 0.835 microg ml(-1)) and thrombin (IC(50) = 9.3 microg ml(-1)) in the presence of antithrombin. In vivo assays demonstrated that at the dose of 1 mg kg(-1), the mollusk HS inhibited thrombus growth in photochemically injured arteries. No bleeding effect, factor XIIa-mediated kallikrein activity, or toxic effect on fibroblast cells was induced by the invertebrate HS at the antithrombotic dose.
Figures




Similar articles
-
Heparin from bovine intestinal mucosa: glycans with multiple sulfation patterns and anticoagulant effects.Thromb Haemost. 2012 May;107(5):903-15. doi: 10.1160/TH-11-07-0518. Epub 2012 Mar 22. Thromb Haemost. 2012. PMID: 22437650
-
An algal sulfated galactan has an unusual dual effect on venous thrombosis due to activation of factor XII and inhibition of the coagulation proteases.Thromb Haemost. 2008 Mar;99(3):531-8. doi: 10.1160/TH07-10-0649. Thromb Haemost. 2008. PMID: 18327401
-
A non-hemorrhagic hybrid heparin/heparan sulfate with anticoagulant potential.Carbohydr Polym. 2014 Jan;99:372-8. doi: 10.1016/j.carbpol.2013.08.063. Epub 2013 Aug 29. Carbohydr Polym. 2014. PMID: 24274520
-
Structure and anticoagulant properties of sulfated glycosaminoglycans from primitive Chordates.An Acad Bras Cienc. 2002 Mar;74(1):105-12. doi: 10.1590/s0001-37652002000100007. An Acad Bras Cienc. 2002. PMID: 11960179 Review.
-
Heparins and heparinoids: occurrence, structure and mechanism of antithrombotic and hemorrhagic activities.Curr Pharm Des. 2004;10(9):951-66. doi: 10.2174/1381612043452758. Curr Pharm Des. 2004. PMID: 15078126 Review.
Cited by
-
Importance of Heparan Sulfate Proteoglycans in Pancreatic Islets and β-Cells.Int J Mol Sci. 2022 Oct 11;23(20):12082. doi: 10.3390/ijms232012082. Int J Mol Sci. 2022. PMID: 36292936 Free PMC article. Review.
-
Methodologies to generate, extract, purify and fractionate yeast ECM for analytical use in proteomics and glycomics.BMC Microbiol. 2014 Oct 25;14:244. doi: 10.1186/s12866-014-0244-0. BMC Microbiol. 2014. PMID: 25344425 Free PMC article.
-
Antioxidant and antiproliferative effect of a glycosaminoglycan extract from Rapana venosa marine snail.PLoS One. 2024 Feb 15;19(2):e0297803. doi: 10.1371/journal.pone.0297803. eCollection 2024. PLoS One. 2024. PMID: 38359063 Free PMC article.
-
Non-Anticoagulant Heparins as Heparanase Inhibitors.Adv Exp Med Biol. 2020;1221:493-522. doi: 10.1007/978-3-030-34521-1_20. Adv Exp Med Biol. 2020. PMID: 32274724 Free PMC article. Review.
-
Antioxidant Activity Derived from Marine Green-Lipped Mussel Perna canaliculus Extracts in Mice.Biomed Res Int. 2021 Aug 6;2021:1622270. doi: 10.1155/2021/1622270. eCollection 2021. Biomed Res Int. 2021. PMID: 34409099 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

