Intramarrow injection of beta-catenin-activated, but not naive mesenchymal stromal cells stimulates self-renewal of hematopoietic stem cells in bone marrow
- PMID: 20054234
- PMCID: PMC2827829
- DOI: 10.3858/emm.2010.42.2.014
Intramarrow injection of beta-catenin-activated, but not naive mesenchymal stromal cells stimulates self-renewal of hematopoietic stem cells in bone marrow
Abstract
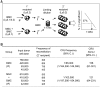
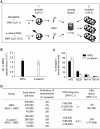
Bone marrow mesenchymal stromal cells (MSCs) have been implicated in the microenvironmental support of hematopoietic stem cells (HSCs) and often co-transplanted with HSCs to facilitate recovery of ablated bone marrows. However, the precise effect of transplanted MSCs on HSC regeneration remains unclear because the kinetics of HSC self-renewal in vivo after co-transplantation has not been monitored. In this study, we examined the effects of intrafemoral injection of MSCs on HSC self-renewal in rigorous competitive repopulating unit (CRU) assays using congenic transplantation models in which stromal progenitors (CFU-F) were ablated by irradiation. Interestingly, naïve MSCs injected into femur contributed to the reconstitution of a stromal niche in the ablated bone marrows, but did not exert a stimulatory effect on the in-vivo self-renewal of co-transplanted HSCs regardless of the transplantation methods. In contrast, HSC self-renewal was four-fold higher in bone marrows intrafemorally injected with beta-catenin-activated MSCs. These results reveal that naive MSCs lack a stimulatory effect on HSC self-renewal in-vivo and that stroma must be activated during recoveries of bone marrows. Stromal targeting of wnt/beta-catenin signals may be a strategy to activate such a stem cell niche for efficient regeneration of bone marrow HSCs.
Figures



References
-
- Adams GB, Scadden DT. A niche opportunity for stem cell therapeutics. Gene Ther. 2008;15:96–99. - PubMed
-
- Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764–2767. - PubMed
-
- Bartsch K, Al-Ali H, Reinhardt A, Franke C, Hudecek M, Kamprad M, Tschiedel S, Cross M, Niederwieser D, Gentilini C. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation. 2009;87:217–221. - PubMed
-
- Boggs DR. The total marrow mass of the mouse: a simplified method of measurement. Am J Hematol. 1984;16:277–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
