Binding of natural and synthetic polyphenols to human dihydrofolate reductase
- PMID: 20054477
- PMCID: PMC2802001
- DOI: 10.3390/ijms10125398
Binding of natural and synthetic polyphenols to human dihydrofolate reductase
Abstract
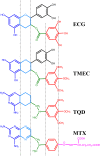
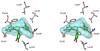
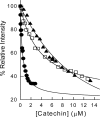
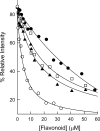
Dihydrofolate reductase (DHFR) is the subject of intensive investigation since it appears to be the primary target enzyme for antifolate drugs. Fluorescence quenching experiments show that the ester bond-containing tea polyphenols (-)-epigallocatechin gallate (EGCG) and (-)-epicatechin gallate (ECG) are potent inhibitors of DHFR with dissociation constants (K(D))of 0.9 and 1.8 microM, respectively, while polyphenols lacking the ester bound gallate moiety [e.g., (-)-epigallocatechin (EGC) and (-)-epicatechin (EC)] did not bind to this enzyme. To avoid stability and bioavailability problems associated with tea catechins we synthesized a methylated derivative of ECG (3-O-(3,4,5-trimethoxybenzoyl)-(-)-epicatechin; TMECG), which effectively binds to DHFR (K(D) = 2.1 microM). In alkaline solution, TMECG generates a stable quinone methide product that strongly binds to the enzyme with a K(D) of 8.2 nM. Quercetin glucuronides also bind to DHFR but its effective binding was highly dependent of the sugar residue, with quercetin-3-xyloside being the stronger inhibitor of the enzyme with a K(D) of 0.6 microM. The finding that natural polyphenols are good inhibitors of human DHFR could explain the epidemiological data on their prophylactic effects for certain forms of cancer and open a possibility for the use of natural and synthetic polyphenols in cancer chemotherapy.
Keywords: antifolates; dihydrofolate reductase; enzyme inhibition; flavonoids; polyphenols; tea catechins.
Figures

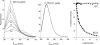


Similar articles
-
Kinetics of the inhibition of bovine liver dihydrofolate reductase by tea catechins: origin of slow-binding inhibition and pH studies.Biochemistry. 2005 May 24;44(20):7512-25. doi: 10.1021/bi050160t. Biochemistry. 2005. PMID: 15895994
-
Factors influencing the antifolate activity of synthetic tea-derived catechins.Molecules. 2013 Jul 16;18(7):8319-41. doi: 10.3390/molecules18078319. Molecules. 2013. PMID: 23863773 Free PMC article.
-
The antifolate activity of tea catechins.Cancer Res. 2005 Mar 15;65(6):2059-64. doi: 10.1158/0008-5472.CAN-04-3469. Cancer Res. 2005. PMID: 15781612
-
Regulation of human dihydrofolate reductase activity and expression.Vitam Horm. 2008;79:267-92. doi: 10.1016/S0083-6729(08)00409-3. Vitam Horm. 2008. PMID: 18804698 Review.
-
Dihydrofolate reductase inhibitors: patent landscape and phases of clinical development (2001-2021).Expert Opin Ther Pat. 2022 Oct;32(10):1079-1095. doi: 10.1080/13543776.2022.2130752. Epub 2022 Oct 7. Expert Opin Ther Pat. 2022. PMID: 36189616 Review.
Cited by
-
In silico design of novel bioactive molecules to treat breast cancer with chlorogenic acid derivatives: a computational and SAR approach.Front Pharmacol. 2023 Dec 12;14:1266833. doi: 10.3389/fphar.2023.1266833. eCollection 2023. Front Pharmacol. 2023. PMID: 38152692 Free PMC article.
-
Bioassay-Guided Fractionation, ESI-MS Scan, Phytochemical Screening, and Antiplasmodial Activity of Afzelia africana.Biochem Res Int. 2022 Apr 13;2022:6895560. doi: 10.1155/2022/6895560. eCollection 2022. Biochem Res Int. 2022. PMID: 35465443 Free PMC article.
-
Dietary polyphenols influence antimetabolite agents: methotrexate, 6-mercaptopurine and 5-fluorouracil in leukemia cell lines.Oncotarget. 2017 Aug 24;8(62):104877-104893. doi: 10.18632/oncotarget.20501. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285220 Free PMC article.
-
Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii.Int J Mol Sci. 2024 Aug 28;25(17):9321. doi: 10.3390/ijms25179321. Int J Mol Sci. 2024. PMID: 39273268 Free PMC article.
-
Computational Drug Repositioning for Chagas Disease Using Protein-Ligand Interaction Profiling.Int J Mol Sci. 2020 Jun 16;21(12):4270. doi: 10.3390/ijms21124270. Int J Mol Sci. 2020. PMID: 32560043 Free PMC article.
References
-
- Mukhtar H, Ahmad N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000;71:1698S–1702S. - PubMed
-
- Fujiki H, Sugunuma M, Imai K, Nakachi K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett. 2002;188:9–13. - PubMed
-
- Hamilton-Miller JMT. Anti-cariogenic properties of tea (Camellia Sinensis) J. Med. Microbiol. 2001;50:299–302. - PubMed
-
- Yam TS, Hamilton-Miller JMT, Shah S. The effect of a component of tea (Camellia Sinensis) on methicillin resistance, PBP2′ synthesis, and beta-lactamase production in Staphylococcus Aureus. J. Antimicrob. Chemother. 1998;42:211–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

