Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts
- PMID: 20054482
- PMCID: PMC2802006
- DOI: 10.3390/ijms10125485
Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts
Abstract
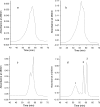
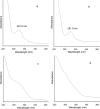
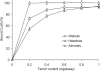
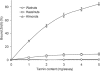
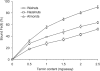
The tannin fractions isolated from hazelnuts, walnuts and almonds were characterised by colorimetric assays and by an SE-HPLC technique. The complexation of Cu(II) and Zn(II) was determined by the reaction with tetramethylmurexide, whereas for Fe(II), ferrozine was employed. The walnut tannins exhibited a significantly weaker reaction with the vanillin/HCl reagent than hazelnut and almond tannins, but the protein precipitation capacity of the walnut fraction was high. The SE-HPLC chromatogram of the tannin fraction from hazelnuts revealed the presence of oligomers with higher molecular weights compared to that of almonds. Copper ions were most effectively chelated by the constituents of the tannin fractions of hazelnuts, walnuts and almonds. At a 0.2 mg/assay addition level, the walnut tannins complexed almost 100% Cu(II). The Fe(II) complexation capacities of the tannin fractions of walnuts and hazelnuts were weaker in comparison to that of the almond tannin fraction, which at a 2.5 mg/assay addition level, bound Fe(II) by approximately 90%. The capacity to chelate Zn(II) was quite varied for the different nut tannin fractions: almond tannins bound as much as 84% Zn(II), whereas the value for walnut tannins was only 8.7%; and for hazelnut tannins, no Zn(II) chelation took place at the levels tested.
Keywords: Cu(II); Fe(II); Zn(II); almonds; hazelnuts; metal chelating capacity; tannins; walnuts.
Figures
References
-
- Halvorsen BL, Holte K, Myhrstad MCW, Barikmo I, Hvattum E, Remberg SF, Wold AB, Haffner K, Baugerod H, Andersen LF, Moskaug JO, Jacobs DR, Blopmhoff R. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002;132:461–471. - PubMed
-
- Blomhoff R, Carlsen MH, Andersen LP, Jacobs DR., Jr Health benefits of nuts: potential role of antioxidants. Brit J Nutr. 2006;96:S52–S60. - PubMed
-
- Yurttas HC, Schafer HW, Warthesen JJ. Antioxidant activity of nontocopherol hazelnut (Corylus spp.) phenolics. J Food Sci. 2000;65:276–280.
-
- Wijeratne SSK, Abou-Zaid MM, Shahidi F. Antioxidant polyphenols in almond and its coproducts. J. Agric. Food Chem. 2006;54:312–318. - PubMed
-
- Alasalvar C, Karamać M, Amarowicz R, Shahidi F. Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J Agric Food Chem. 2006;54:4826–4832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

