The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells
- PMID: 20054641
- PMCID: PMC3295576
- DOI: 10.1007/s10549-009-0716-3
The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells
Abstract
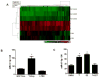
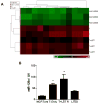
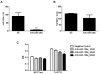
Resistance to endocrine therapy agents has presented a clinical obstacle in the treatment of hormone-dependent breast cancer. Our laboratory has initiated a study of microRNA regulation of signaling pathways that may result in breast cancer progression on aromatase inhibitors (AI). Microarray analysis of hormone refractory cell lines identified 115 differentially regulated microRNAs, of which 49 microRNAs were believed to be hormone-responsive. A group of microRNAs were inversely expressed in the AI-resistant lines versus LTEDaro and tamoxifen-resistant. We focused our work on hsa-miR-128a which was hormone-responsive and selectively up-regulated in the letrozole-resistant cell lines. Human miR-128a was predicted to target the TGFβ signaling pathway and indeed sensitivity to TGFβ was compromised in the letrozole-resistant cells, as compared to parental MCF-7aro. Human miR-128a was shown to negatively target TGFβRI protein expression by binding to the 3'UTR region of the gene. Inhibition of endogenous miR-128a resulted in resensitization of the letrozole-resistant lines to TGFβ growth inhibitory effects. These data suggest that the hormone-responsive miR-128a can modulate TGFβ signaling and survival of the letrozole-resistant cell lines. To our knowledge, this is the first study to address the role of microRNA regulation as well as TGFβ signaling in AI-resistant breast cancer cell lines. We believe that in addition to estrogen-modulation of gene expression, hormone-regulated microRNAs may provide an additional level of post-transcriptional regulation of signaling pathways critically involved in breast cancer progression and AI-resistance.
Figures



Similar articles
-
MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer.Breast Cancer Res. 2015 Jan 30;17(1):13. doi: 10.1186/s13058-015-0515-1. Breast Cancer Res. 2015. PMID: 25633049 Free PMC article.
-
Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines.J Steroid Biochem Mol Biol. 2010 Feb 28;118(4-5):277-82. doi: 10.1016/j.jsbmb.2009.10.011. Epub 2009 Nov 6. J Steroid Biochem Mol Biol. 2010. PMID: 19897035 Free PMC article.
-
GP88 (PC-Cell Derived Growth Factor, progranulin) stimulates proliferation and confers letrozole resistance to aromatase overexpressing breast cancer cells.BMC Cancer. 2011 Jun 9;11:231. doi: 10.1186/1471-2407-11-231. BMC Cancer. 2011. PMID: 21658239 Free PMC article.
-
Letrozole: a pharmacoeconomic review of its use in postmenopausal women with breast cancer.Pharmacoeconomics. 2006;24(5):495-517. doi: 10.2165/00019053-200624050-00007. Pharmacoeconomics. 2006. PMID: 16706574 Review.
-
Identification of miRNAs as biomarkers for acquired endocrine resistance in breast cancer.Mol Cell Endocrinol. 2017 Nov 15;456:76-86. doi: 10.1016/j.mce.2017.02.004. Epub 2017 Feb 3. Mol Cell Endocrinol. 2017. PMID: 28163101 Review.
Cited by
-
TFPI alpha and beta regulate mRNAs and microRNAs involved in cancer biology and in the immune system in breast cancer cells.PLoS One. 2012;7(10):e47184. doi: 10.1371/journal.pone.0047184. Epub 2012 Oct 5. PLoS One. 2012. PMID: 23071754 Free PMC article.
-
miRNAs and estrogen action.Trends Endocrinol Metab. 2012 May;23(5):223-33. doi: 10.1016/j.tem.2012.03.002. Epub 2012 Apr 11. Trends Endocrinol Metab. 2012. PMID: 22503553 Free PMC article. Review.
-
Global microRNA expression profiling of high-risk ER+ breast cancers from patients receiving adjuvant tamoxifen mono-therapy: a DBCG study.PLoS One. 2012;7(5):e36170. doi: 10.1371/journal.pone.0036170. Epub 2012 May 18. PLoS One. 2012. PMID: 22623953 Free PMC article.
-
MicroRNA-mediated drug resistance in breast cancer.Clin Epigenetics. 2011 Aug;2(2):171-185. doi: 10.1007/s13148-011-0040-8. Epub 2011 Jun 27. Clin Epigenetics. 2011. PMID: 21949547 Free PMC article.
-
Roles for miRNAs in endocrine resistance in breast cancer.Endocr Relat Cancer. 2015 Oct;22(5):R279-300. doi: 10.1530/ERC-15-0355. Endocr Relat Cancer. 2015. PMID: 26346768 Free PMC article. Review.
References
-
- Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–32. - PubMed
-
- Yue W, Santen RJ, Wang JP, et al. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol. 2003;86(3-5):477–86. - PubMed
-
- Santner SJ, Chen S, Zhou D, Korsunsky Z, Martel J, Santen RJ. Effect of androstenedione on growth of untransfected and aromatase-transfected MCF-7 cells in culture. J Steroid Biochem Mol Biol. 1993;44(4-6):611–6. - PubMed
-
- Yue W, Wang JP, Hamilton CJ, Demers LM, Santen RJ. In situ aromatization enhances breast tumor estradiol levels and cellular proliferation. Cancer Res. 1998;58(5):927–32. - PubMed
-
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351(9114):1451–67. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

