Transcriptional regulation of the Oct4 gene, a master gene for pluripotency
- PMID: 20054811
- PMCID: PMC3418322
- DOI: 10.14670/HH-25.405
Transcriptional regulation of the Oct4 gene, a master gene for pluripotency
Abstract
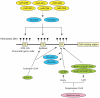
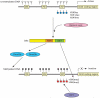
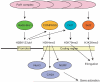
Oct4 is one of the most important transcription factors required to maintain an undifferentiated state (self-renewal) and pluripotency of human and mouse embryonic stem (ES) cells as well as early embryonic cells. In addition, Oct4 is the only known transcription factor that has to be exogenously introduced into differentiated cells to make induced pluripotent stem (iPS) cells. Therefore, it is of great importance to understand how Oct4 transcription is regulated in ES cells and embryos and how it becomes activated during iPS cell formation. In this article, we will review the regulation of the mouse Oct4 gene from the viewpoint of DNA methylation, binding of orphan nuclear receptors, histone modifications and synergistic effects with other pluripotency factors. We will also raise several key questions that need to be addressed in future work to improve our understanding of Oct4 gene regulation and its essential role in self-renewal and pluripotency.
Figures
Similar articles
-
Ethyl-p-methoxycinnamate enhances oct4 expression and reinforces pluripotency through the NF-κB signaling pathway.Biochem Pharmacol. 2020 Jul;177:113984. doi: 10.1016/j.bcp.2020.113984. Epub 2020 Apr 18. Biochem Pharmacol. 2020. PMID: 32311348
-
A novel SALL4/OCT4 transcriptional feedback network for pluripotency of embryonic stem cells.PLoS One. 2010 May 21;5(5):e10766. doi: 10.1371/journal.pone.0010766. PLoS One. 2010. PMID: 20505821 Free PMC article.
-
Nanog, Oct4 and Tet1 interplay in establishing pluripotency.Sci Rep. 2016 May 5;6:25438. doi: 10.1038/srep25438. Sci Rep. 2016. PMID: 27146218 Free PMC article.
-
Role of Oct4 in maintaining and regaining stem cell pluripotency.Stem Cell Res Ther. 2010 Dec 14;1(5):39. doi: 10.1186/scrt39. Stem Cell Res Ther. 2010. PMID: 21156086 Free PMC article. Review.
-
Regulation of the protein stability and transcriptional activity of OCT4 in stem cells.Adv Biol Regul. 2021 Jan;79:100777. doi: 10.1016/j.jbior.2020.100777. Epub 2020 Dec 29. Adv Biol Regul. 2021. PMID: 33451972 Review.
Cited by
-
Phospholipid--driven gene regulation.FEBS Lett. 2013 Apr 17;587(8):1238-46. doi: 10.1016/j.febslet.2013.01.004. Epub 2013 Jan 16. FEBS Lett. 2013. PMID: 23333623 Free PMC article. Review.
-
All-trans Retinoic Acid as a Versatile Cytosolic Signal Modulator Mediated by CRABP1.Int J Mol Sci. 2019 Jul 24;20(15):3610. doi: 10.3390/ijms20153610. Int J Mol Sci. 2019. PMID: 31344789 Free PMC article. Review.
-
Alternative Splicing in Neurogenesis and Brain Development.Front Mol Biosci. 2018 Feb 12;5:12. doi: 10.3389/fmolb.2018.00012. eCollection 2018. Front Mol Biosci. 2018. PMID: 29484299 Free PMC article. Review.
-
A comparison of pluripotency and differentiation status of four mesenchymal adult stem cells.Mol Biol Rep. 2013 May;40(5):3693-703. doi: 10.1007/s11033-012-2445-7. Epub 2012 Dec 29. Mol Biol Rep. 2013. PMID: 23275202
-
Chemical screen for epigenetic barriers to single allele activation of Oct4.Stem Cell Res. 2019 Jul;38:101470. doi: 10.1016/j.scr.2019.101470. Epub 2019 May 24. Stem Cell Res. 2019. PMID: 31170660 Free PMC article.
References
-
- Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. - PubMed
-
- Boiani M, Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2005;6:872–884. - PubMed
-
- Burdon T, Chambers I, Stracey C, Niwa H, Smith A. Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs. 1999;165:131–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
