The solution structure of the Mg2+ form of soybean calmodulin isoform 4 reveals unique features of plant calmodulins in resting cells
- PMID: 20054830
- PMCID: PMC2866273
- DOI: 10.1002/pro.325
The solution structure of the Mg2+ form of soybean calmodulin isoform 4 reveals unique features of plant calmodulins in resting cells
Abstract
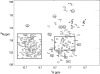
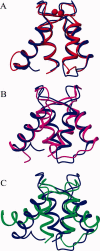
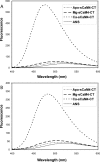
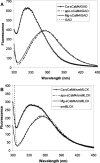
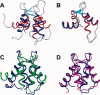
Soybean calmodulin isoform 4 (sCaM4) is a plant calcium-binding protein, regulating cellular responses to the second messenger Ca(2+). We have found that the metal ion free (apo-) form of sCaM4 possesses a half unfolded structure, with the N-terminal domain unfolded and the C-terminal domain folded. This result was unexpected as the apo-forms of both soybean calmodulin isoform 1 (sCaM1) and mammalian CaM (mCaM) are fully folded. Because of the fact that free Mg(2+) ions are always present at high concentrations in cells (0.5-2 mM), we suggest that Mg(2+) should be bound to sCaM4 in nonactivated cells. CD studies revealed that in the presence of Mg(2+) the initially unfolded N-terminal domain of sCaM4 folds into an alpha-helix-rich structure, similar to the Ca(2+) form. We have used the NMR backbone residual dipolar coupling restraints (1)D(NH), (1)D(C alpha H alpha), and (1)D(C'C alpha) to determine the solution structure of the N-terminal domain of Mg(2+)-sCaM4 (Mg(2+)-sCaM4-NT). Compared with the known structure of Ca(2+)-sCaM4, the structure of the Mg(2+)-sCaM4-NT does not fully open the hydrophobic pocket, which was further confirmed by the use of the fluorescent probe ANS. Tryptophan fluorescence experiments were used to study the interactions between Mg(2+)-sCaM4 and CaM-binding peptides derived from smooth muscle myosin light chain kinase and plant glutamate decarboxylase. These results suggest that Mg(2+)-sCaM4 does not bind to Ca(2+)-CaM target peptides and therefore is functionally similar to apo-mCaM. The Mg(2+)- and apo-structures of the sCaM4-NT provide unique insights into the structure and function of some plant calmodulins in resting cells.
Figures

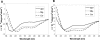
Similar articles
-
The solution structures of two soybean calmodulin isoforms provide a structural basis for their selective target activation properties.J Biol Chem. 2008 May 23;283(21):14619-28. doi: 10.1074/jbc.M801398200. Epub 2008 Mar 17. J Biol Chem. 2008. PMID: 18347016
-
Structural studies of soybean calmodulin isoform 4 bound to the calmodulin-binding domain of tobacco mitogen-activated protein kinase phosphatase-1 provide insights into a sequential target binding mode.J Biol Chem. 2009 Oct 9;284(41):28292-28305. doi: 10.1074/jbc.M109.025080. Epub 2009 Aug 10. J Biol Chem. 2009. PMID: 19667066 Free PMC article.
-
Activation of smooth muscle myosin light chain kinase by calmodulin. Role of LYS(30) and GLY(40).J Biol Chem. 2002 Feb 22;277(8):6550-8. doi: 10.1074/jbc.M111404200. Epub 2001 Dec 17. J Biol Chem. 2002. PMID: 11748245
-
Structure, dynamics and interaction with kinase targets: computer simulations of calmodulin.Biochim Biophys Acta. 2004 Mar 11;1697(1-2):289-300. doi: 10.1016/j.bbapap.2003.11.032. Biochim Biophys Acta. 2004. PMID: 15023369 Review.
-
The Merck Frosst Award Lecture 1994. Calmodulin: a versatile calcium mediator protein.Biochem Cell Biol. 1994 Sep-Oct;72(9-10):357-76. Biochem Cell Biol. 1994. PMID: 7605608 Review.
Cited by
-
Structural basis for the activation of platelet integrin αIIbβ3 by calcium- and integrin-binding protein 1.J Am Chem Soc. 2012 Feb 29;134(8):3864-72. doi: 10.1021/ja2111306. Epub 2012 Feb 16. J Am Chem Soc. 2012. PMID: 22283712 Free PMC article.
-
Studying Peptide-Metal Ion Complex Structures by Solution-State NMR.Int J Mol Sci. 2022 Dec 15;23(24):15957. doi: 10.3390/ijms232415957. Int J Mol Sci. 2022. PMID: 36555599 Free PMC article. Review.
-
Structural basis for Ca2+-induced activation and dimerization of estrogen receptor α by calmodulin.J Biol Chem. 2012 Mar 16;287(12):9336-44. doi: 10.1074/jbc.M111.334797. Epub 2012 Jan 23. J Biol Chem. 2012. PMID: 22275375 Free PMC article.
-
Solution structures of Ca2+-CIB1 and Mg2+-CIB1 and their interactions with the platelet integrin alphaIIb cytoplasmic domain.J Biol Chem. 2011 May 13;286(19):17181-92. doi: 10.1074/jbc.M110.179028. Epub 2011 Mar 9. J Biol Chem. 2011. PMID: 21388953 Free PMC article.
-
Comparing the calcium binding abilities of two soybean calmodulins: towards understanding the divergent nature of plant calmodulins.Plant Cell. 2013 Nov;25(11):4512-24. doi: 10.1105/tpc.113.113183. Epub 2013 Nov 19. Plant Cell. 2013. PMID: 24254124 Free PMC article.
References
-
- Yamniuk AP, Vogel HJ. Calmodulin's flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol Biotechnol. 2004;27:33–57. - PubMed
-
- Barbato G, Ikura M, Kay LE, Pastor RW, Bax A. Backbone dynamics of calmodulin studied by N-15 relaxation using inverse detected 2-dimensional NMR-spectroscopy—the central helix is flexible. Biochemistry. 1992;31:5269–5278. - PubMed
-
- Henikoff S, Greene EA, Pietrokovski S, Bork P, Attwood TK, Hood L. Gene families: the taxonomy of protein paralogs and chimeras. Science. 1997;278:609–614. - PubMed
-
- Gifford JL, Walsh MP, Vogel HJ. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem J. 2007;405:199–221. - PubMed
-
- Wilson MA, Brunger AT. The 1.0 angstrom crystal structure of Ca2+-bound calmodulan analysis of disorder and implications for functionally relevant plasticity. J Mol Biol. 2000;301:1237–1256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

