Subcellular dynamics and protein conformation fluctuations measured by Fourier imaging correlation spectroscopy
- PMID: 20055672
- PMCID: PMC4833490
- DOI: 10.1146/annurev.physchem.012809.103500
Subcellular dynamics and protein conformation fluctuations measured by Fourier imaging correlation spectroscopy
Abstract
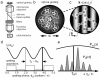
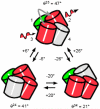
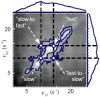
Novel high signal-to-noise spectroscopic experiments that probe the dynamics of microscopic objects have the potential to reveal complex intracellular biochemical mechanisms, or the slow relaxations of soft matter systems. This article reviews the implementation of Fourier imaging correlation spectroscopy (FICS), a phase-selective approach to fluorescence fluctuation spectroscopy that employs a unique route to elevate signal levels while acquiring detailed information about molecular coordinate trajectories. The review demonstrates the broad applicability of FICS by discussing two recent studies. The dynamics of Saccharomyces cerevisiae yeast mitochondria are characterized with FICS and provide detailed information about the influence of specific cytoskeletal elements on the movement of this organelle. In another set of experiments, polarization-modulated FICS captures conformational dynamics and molecular translational dynamics of the fluorescent protein DsRed, and analyses by four-point correlation and joint distribution functions of the corresponding data reveal statistically meaningful pathways of DsRed switching between different optical conformations.
Figures


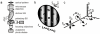
Similar articles
-
Fourier imaging correlation spectroscopy for cellular structure-function.Methods Cell Biol. 2008;90:117-37. doi: 10.1016/S0091-679X(08)00806-6. Methods Cell Biol. 2008. PMID: 19195548
-
II. Kinetic pathways of switching optical conformations in DsRed by 2D Fourier imaging correlation spectroscopy.J Phys Chem B. 2009 May 14;113(19):6854-60. doi: 10.1021/jp901542b. J Phys Chem B. 2009. PMID: 19368361 Free PMC article.
-
I. Conformational dynamics of biological macromolecules by polarization-modulated Fourier imaging correlation spectroscopy.J Phys Chem B. 2009 May 14;113(19):6847-53. doi: 10.1021/jp8093116. J Phys Chem B. 2009. PMID: 19368366 Free PMC article.
-
Fluorescence spectroscopy for revealing mechanisms in biology: Strengths and pitfalls.J Biosci. 2018 Jul;43(3):555-567. J Biosci. 2018. PMID: 30002272 Review.
-
Revealing time bunching effect in single-molecule enzyme conformational dynamics.Phys Chem Chem Phys. 2011 Apr 21;13(15):6734-49. doi: 10.1039/c0cp02860f. Epub 2011 Mar 15. Phys Chem Chem Phys. 2011. PMID: 21409227 Review.
Cited by
-
Using Multiorder Time-Correlation Functions (TCFs) To Elucidate Biomolecular Reaction Pathways from Microsecond Single-Molecule Fluorescence Experiments.J Phys Chem B. 2016 Dec 29;120(51):13003-13016. doi: 10.1021/acs.jpcb.6b08449. Epub 2016 Dec 19. J Phys Chem B. 2016. PMID: 27992233 Free PMC article.
-
Using microsecond single-molecule FRET to determine the assembly pathways of T4 ssDNA binding protein onto model DNA replication forks.Proc Natl Acad Sci U S A. 2017 May 2;114(18):E3612-E3621. doi: 10.1073/pnas.1619819114. Epub 2017 Apr 17. Proc Natl Acad Sci U S A. 2017. PMID: 28416680 Free PMC article.
-
Biological physics in México: Review and new challenges.J Biol Phys. 2011 Mar;37(2):167-84. doi: 10.1007/s10867-011-9218-8. Epub 2011 Feb 11. J Biol Phys. 2011. PMID: 22379227 Free PMC article.
References
-
- Yang H, Luo G, Karnchanaphanurach P, Louie T-M, Rech I, Cova S, Xun L, Xie XS. Protein conformational dynamics probed by single-molecule electron transfer. Science. 2003;302:262–266. - PubMed
-
- Weiss S. Measuring conformational dynamics of biomolecules by single molecule fluorescence spectroscopy. Nat. Struct. Biol. 2000;7:724–729. - PubMed
-
- Grassman TJ, Knowles MK, Marcus AH. Structure and dynamics of fluorescently labeled complex fluids by fourier imaging correlation spectroscopy. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000;62(6 Pt B):8245–57. - PubMed
-
- Dixit R, Cyr R. Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J. 2003;36(2):280–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

