Metabotropic glutamate receptors: physiology, pharmacology, and disease
- PMID: 20055706
- PMCID: PMC2904507
- DOI: 10.1146/annurev.pharmtox.011008.145533
Metabotropic glutamate receptors: physiology, pharmacology, and disease
Abstract
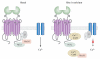
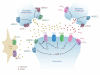
The metabotropic glutamate receptors (mGluRs) are family C G-protein-coupled receptors that participate in the modulation of synaptic transmission and neuronal excitability throughout the central nervous system. The mGluRs bind glutamate within a large extracellular domain and transmit signals through the receptor protein to intracellular signaling partners. A great deal of progress has been made in determining the mechanisms by which mGluRs are activated, proteins with which they interact, and orthosteric and allosteric ligands that can modulate receptor activity. The widespread expression of mGluRs makes these receptors particularly attractive drug targets, and recent studies continue to validate the therapeutic utility of mGluR ligands in neurological and psychiatric disorders such as Alzheimer's disease, Parkinson's disease, anxiety, depression, and schizophrenia.
Figures


References
-
- Pin JP, Galvez T, Prezeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98(3):325–54. - PubMed
-
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407(6807):971–77. - PubMed
-
- Jingami H, Nakanishi S, Morikawa K. Structure of the metabotropic glutamate receptor. Curr. Opin. Neurobiol. 2003;13(3):271–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

